Basic Information
| Drug ID | DDPD00749 |

|
| Drug Name | Etodolac | |
| Molecular Weight | 287.3535 | |
| Molecular Formula | C17H21NO3 | |
| CAS Number | 41340-25-4 | |
| SMILES | CCC1=C2NC3=C(CCOC3(CC)CC(O)=O)C2=CC=C1 | |
| External Links | ||
| DRUGBANK | DB00749 | |
| PubChem Compound | 3308 | |
| PDR | 712 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
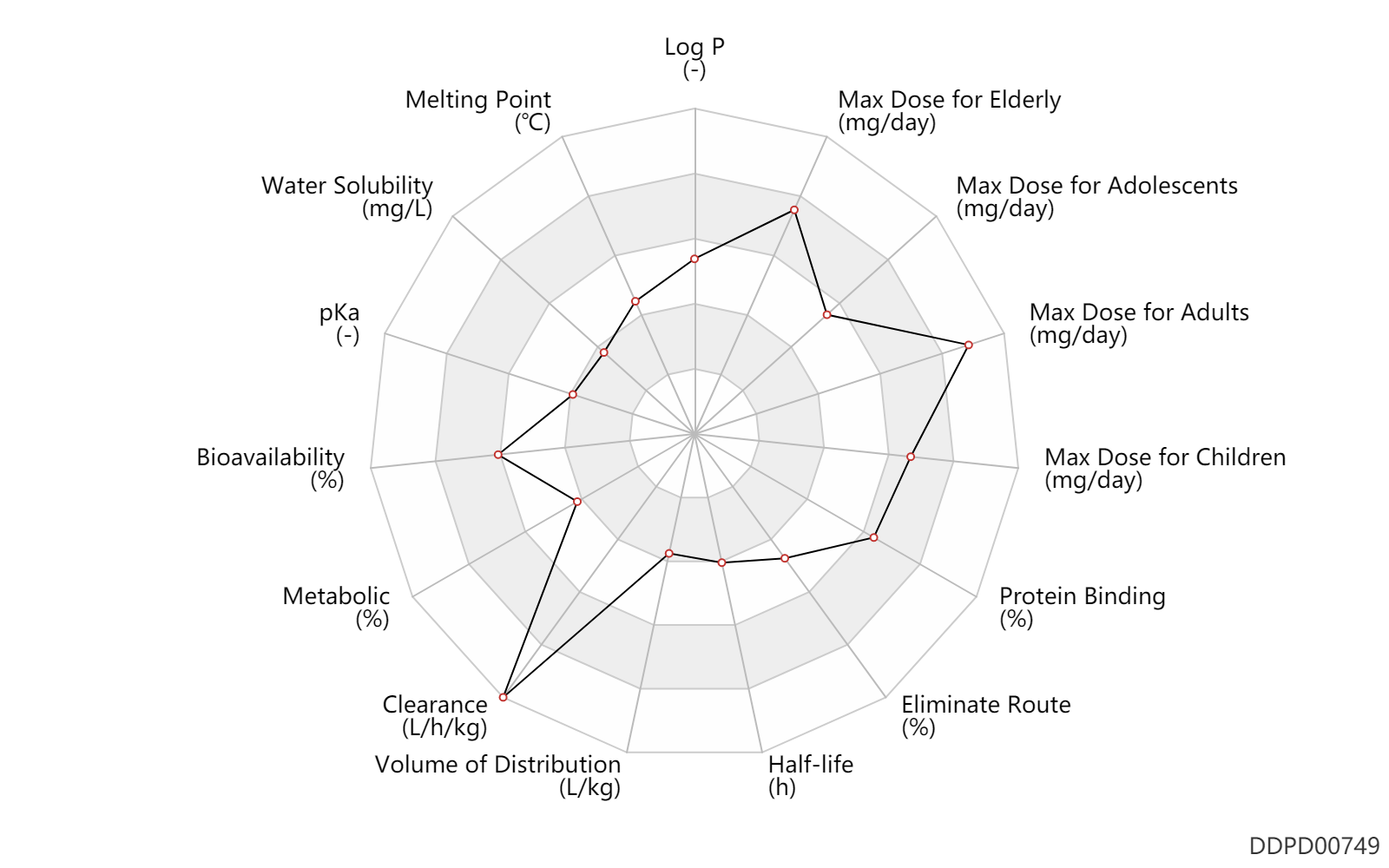
| Log P | 2.5 | - | 2.5 | - | DRUGBANK |
| Melting Point | 146.5 | ℃ | 146.5 | ℃ | PhysProp |
| Water Solubility | 16.0 | mg/L | 16 | mg/L | DRUGBANK |
| pKa | 4.65 | - | 4.65 | - | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 80.0 | % | >80 | % | Tablet, PO, oral; Capsule, PO, Oral; | DRUGBANK |
| Metabolic | 1.0 | % | 1 | % | Urinary excretion; Unchanged drug; | DRUGBANK | Metabolic | 13.0 | % | 13 | % | Urinary excretion; | DRUGBANK | Metabolic | 5.0 | % | 5 | % | Urinary excretion; | DRUGBANK | Metabolic | 20.0 | % | 20 | % | Urinary excretion; | DRUGBANK | Metabolic | 16.0 | % | 16 | % | Faeces excretion; | DRUGBANK |
| Clearance | 2.9 | L/h/kg | 49.1 | ml/min/kg | Total clearance; PO, oral; normal,healthy; adults; | DRUGBANK | Clearance | 3.0 | L/h/kg | 49.4 | ml/min/kg | normal,healthy; Male, men; | DRUGBANK | Clearance | 2.1 | L/h/kg | 35.7 | ml/min/kg | normal,healthy; Female, women; | DRUGBANK | Clearance | 2.7 | L/h/kg | 45.7 | ml/min/kg | Elderly; | DRUGBANK | Clearance | 3.5 | L/h/kg | 58.3 | ml/min/kg | RD, renal impairment, Renal disease,including uremia; | DRUGBANK | Clearance | 2.5 | L/h/kg | 42.0 | ml/min/kg | hepatopathy,LD; | DRUGBANK |
| Volume of Distribution | 0.39 | L/kg | 390.0 | mL/kg | DRUGBANK | |
| Half-life | 7.3 | h | 7.3±4 | h | terminal half-life; | DRUGBANK | Half-life | 0.71 | h | 0.71±0.5 | h | distribution half-life; | DRUGBANK |
| Eliminate Route | 16.0 | % | 16 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 72.0 | % | 72 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 1.0 | % | ~1 | % | Urinary excretion; Unchanged drug; | DRUGBANK |
| Protein Binding | 99.0 | % | >99 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 400.0 | mg/day | 400 | mg/day | Tablet,PO,oral | Etodolac Capsules and Tablets | etodolac | PDR |
| Max dose for children | 600.0 | mg/day | 600 | mg/day | Tablet,PO,oral | Etodolac Capsules and Tablets | etodolac | PDR |
| Max dose for children | 800.0 | mg/day | 800 | mg/day | Tablet,PO,oral | Etodolac Capsules and Tablets | etodolac | PDR |
| Max dose for children | 1000.0 | mg/day | 1000 | mg/day | Tablet,PO,oral | Etodolac Capsules and Tablets | etodolac | PDR |
| Max dose for adolescents | 400.0 | mg/day | 400 | mg/day | Tablet,PO,oral | Etodolac Capsules and Tablets | etodolac | PDR |
| Max dose for adolescents | 600.0 | mg/day | 600 | mg/day | Tablet,PO,oral | Etodolac Capsules and Tablets | etodolac | PDR |
| Max dose for adolescents | 800.0 | mg/day | 800 | mg/day | Tablet,PO,oral | Etodolac Capsules and Tablets | etodolac | PDR |
| Max dose for adolescents | 1000.0 | mg/day | 1000 | mg/day | Tablet,PO,oral | Etodolac Capsules and Tablets | etodolac | PDR |
| Max dose for adults | 1200.0 | mg/day | 1200 | mg/day | PO, oral | Etodolac Capsules and Tablets | etodolac | PDR |
| Max dose for elderly | 1200.0 | mg/day | 1200 | mg/day | PO, oral | Etodolac Capsules and Tablets | etodolac | PDR |