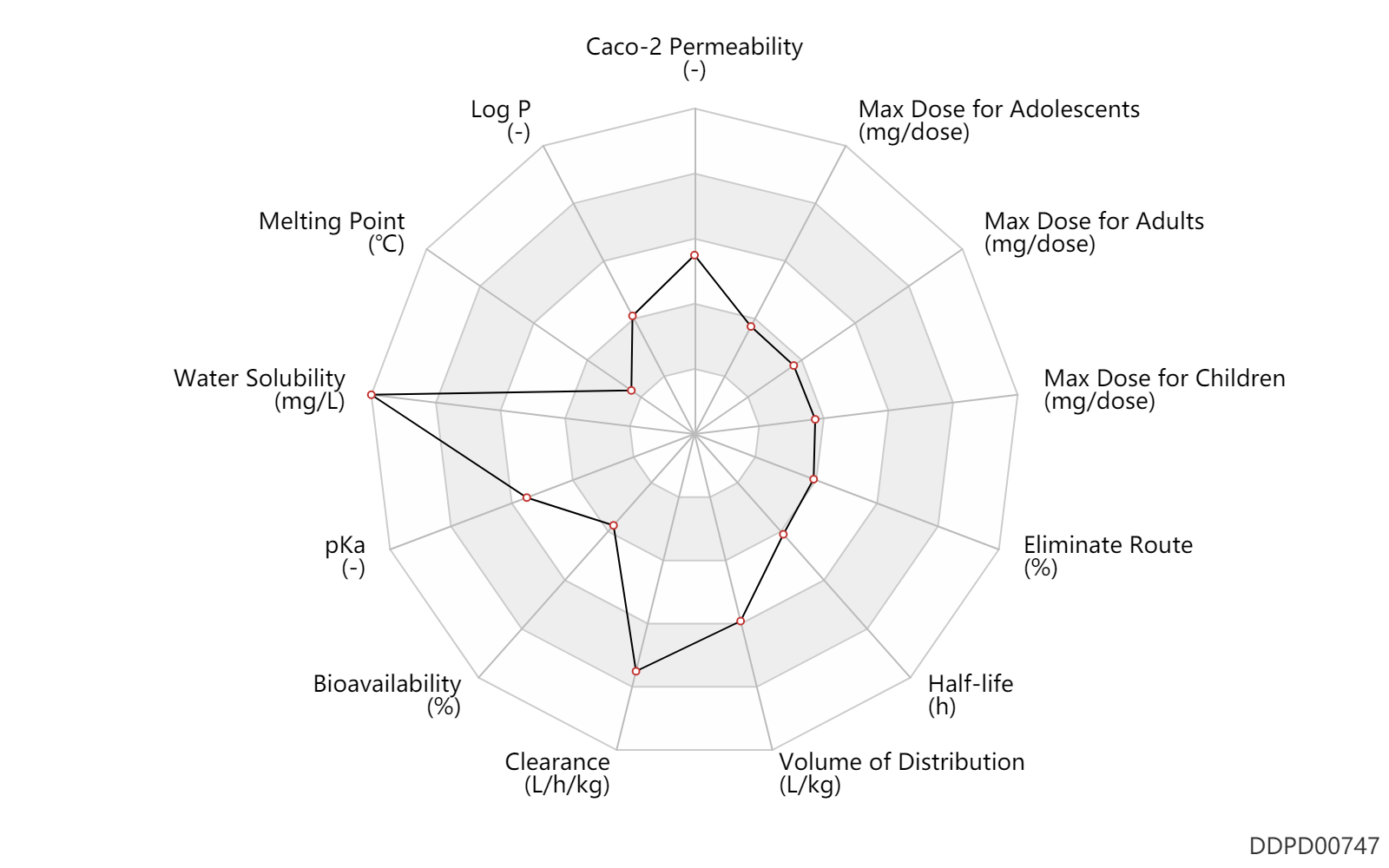
| Max dose for infants |
0.15 |
mg/dose |
0.15 |
mg/dose |
subcutaneous injection, SC;intravenous injection, IV;IM,intramuscular injection; |
Scopolamine |
scopolamine |
PDR |
| Max dose for infants |
3.0 |
drop/day |
3 |
drop/day |
Vaginal Administration |
Scopolamine |
scopolamine |
PDR |
| Max dose for children |
0.6 |
mg/dose |
0.6 |
mg/dose |
subcutaneous injection, SC;intravenous injection, IV;IM,intramuscular injection; |
Scopolamine |
scopolamine |
PDR |
| Max dose for children |
3.0 |
drop/day |
3 |
drop/day |
Vaginal Administration |
Scopolamine |
scopolamine |
PDR |
| Max dose for children |
0.3 |
mg/dose |
0.3 |
mg/dose |
subcutaneous injection, SC;intravenous injection, IV;IM,intramuscular injection; |
Scopolamine |
scopolamine |
PDR |
| Max dose for children |
3.0 |
drop/day |
3 |
drop/day |
Vaginal Administration |
Scopolamine |
scopolamine |
PDR |
| Max dose for children |
0.15 |
mg/dose |
0.15 |
mg/dose |
subcutaneous injection, SC;intravenous injection, IV;IM,intramuscular injection; |
Scopolamine |
scopolamine |
PDR |
| Max dose for children |
3.0 |
drop/day |
3 |
drop/day |
Vaginal Administration |
Scopolamine |
scopolamine |
PDR |
| Max dose for adolescents |
0.6 |
mg/dose |
0.6 |
mg/dose |
subcutaneous injection, SC;intravenous injection, IV;IM,intramuscular injection; |
Scopolamine |
scopolamine |
PDR |
| Max dose for adolescents |
3.0 |
drop/day |
3 |
drop/day |
Vaginal Administration |
Scopolamine |
scopolamine |
PDR |
| Max dose for adults |
2.4 |
mg/dose |
2.4 |
mg/dose |
subcutaneous injection, SC;intravenous injection, IV;IM,intramuscular injection; |
Scopolamine |
scopolamine |
PDR |
| Max dose for adults |
8.0 |
drop/day |
8 |
drop/day |
Vaginal Administration |
Scopolamine |
scopolamine |
PDR |
| Max dose for adults |
1.0 |
patch/dose |
1 |
patch/dose |
Transdermal preparations |
Scopolamine |
scopolamine |
PDR |
| Max dose for geriatric |
2.4 |
mg/dose |
2.4 |
mg/dose |
subcutaneous injection, SC;intravenous injection, IV;IM,intramuscular injection; |
Scopolamine |
scopolamine |
PDR |
| Max dose for geriatric |
8.0 |
drop/day |
8 |
drop/day |
Vaginal Administration |
Scopolamine |
scopolamine |
PDR |
| Max dose for geriatric |
1.0 |
patch/dose |
1 |
patch/dose |
Transdermal preparations |
Scopolamine |
scopolamine |
PDR |