Basic Information
| Drug ID | DDPD00737 |

|
| Drug Name | Meclizine | |
| Molecular Weight | 390.948 | |
| Molecular Formula | C25H27ClN2 | |
| CAS Number | 569-65-3 | |
| SMILES | CC1=CC(CN2CCN(CC2)C(C2=CC=CC=C2)C2=CC=C(Cl)C=C2)=CC=C1 | |
| External Links | ||
| DRUGBANK | DB00737 | |
| T3DB | T3D2874 | |
| PubChem Compound | 4034 | |
| PDR | 1822 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
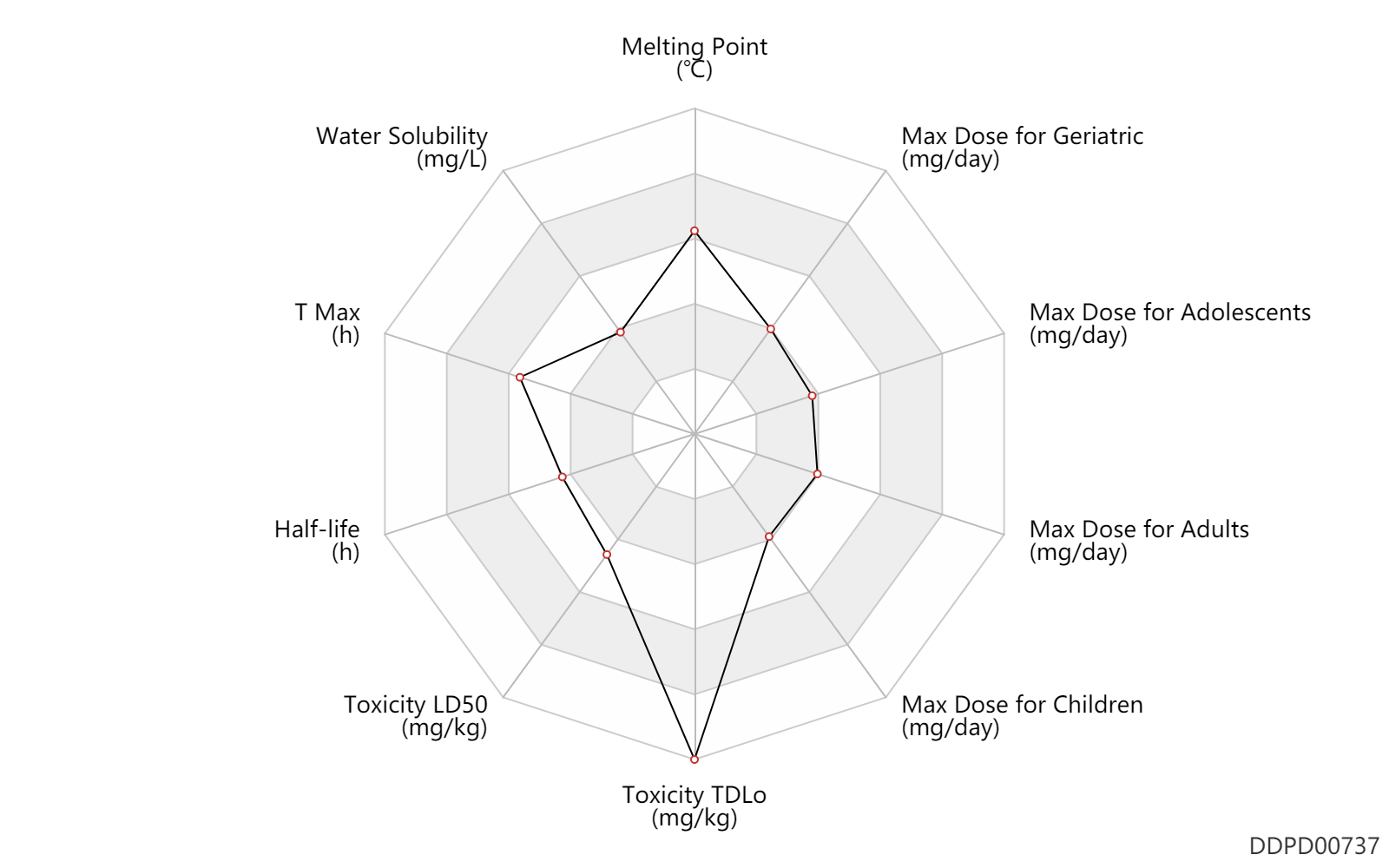
| Melting Point | 220.5 | ℃ | 217-224 | ℃ | U.S. Patent 2,709,169. |
| Water Solubility | 1000.0 | mg/L | 0.1 | g/100ml | BONAMINE? Product Monograph - McNeil Consumer Healthcare, division of Johnson & Johnson Inc. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| T Max | 3.0 | h | 3(1.5-6) | h | PO, oral; | DRUGBANK |
| Half-life | 5.5 | h | ~5-6 | h | elimination half-life; | DRUGBANK |
| Toxicity LD50 | 1600.0 | mg/kg | 1600.0 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 625.0 | mg/kg | 625.0 | mg/kg | Intraperitoneal, IP; mouse; | DRUGBANK | Toxicity LD50 | 1600.0 | mg/kg | 1600.0 | mg/kg | PO, oral; | T3DB | Toxicity LD50 | 659.0 | mg/kg | 659.0 | mg/kg | Intraperitoneal, IP; | T3DB |
| Toxicity TDLo | 800.0 | mg/kg | 800.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 50.0 | mg/day | 50 | mg/day | PO, oral | Antivert | meclizine hydrochloride | PDR |
| Max dose for adolescents | 50.0 | mg/day | 50 | mg/day | PO, oral | Antivert | meclizine hydrochloride | PDR |
| Max dose for adults | 100.0 | mg/day | 100 | mg/day | PO, oral | Antivert | meclizine hydrochloride | PDR |
| Max dose for adults | 50.0 | mg/day | 50 | mg/day | PO, oral | Antivert | meclizine hydrochloride | PDR |
| Max dose for geriatric | 100.0 | mg/day | 100 | mg/day | PO, oral | Antivert | meclizine hydrochloride | PDR |
| Max dose for geriatric | 50.0 | mg/day | 50 | mg/day | PO, oral | Antivert | meclizine hydrochloride | PDR |