Basic Information
| Drug ID | DDPD00710 |

|
| Drug Name | Ibandronate | |
| Molecular Weight | 319.2289 | |
| Molecular Formula | C9H23NO7P2 | |
| CAS Number | 114084-78-5 | |
| SMILES | CCCCCN(C)CCC(O)(P(O)(O)=O)P(O)(O)=O | |
| External Links | ||
| DRUGBANK | DB00710 | |
| T3DB | T3D2667 | |
| PubChem Compound | 60852 | |
| PDR | 2108 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
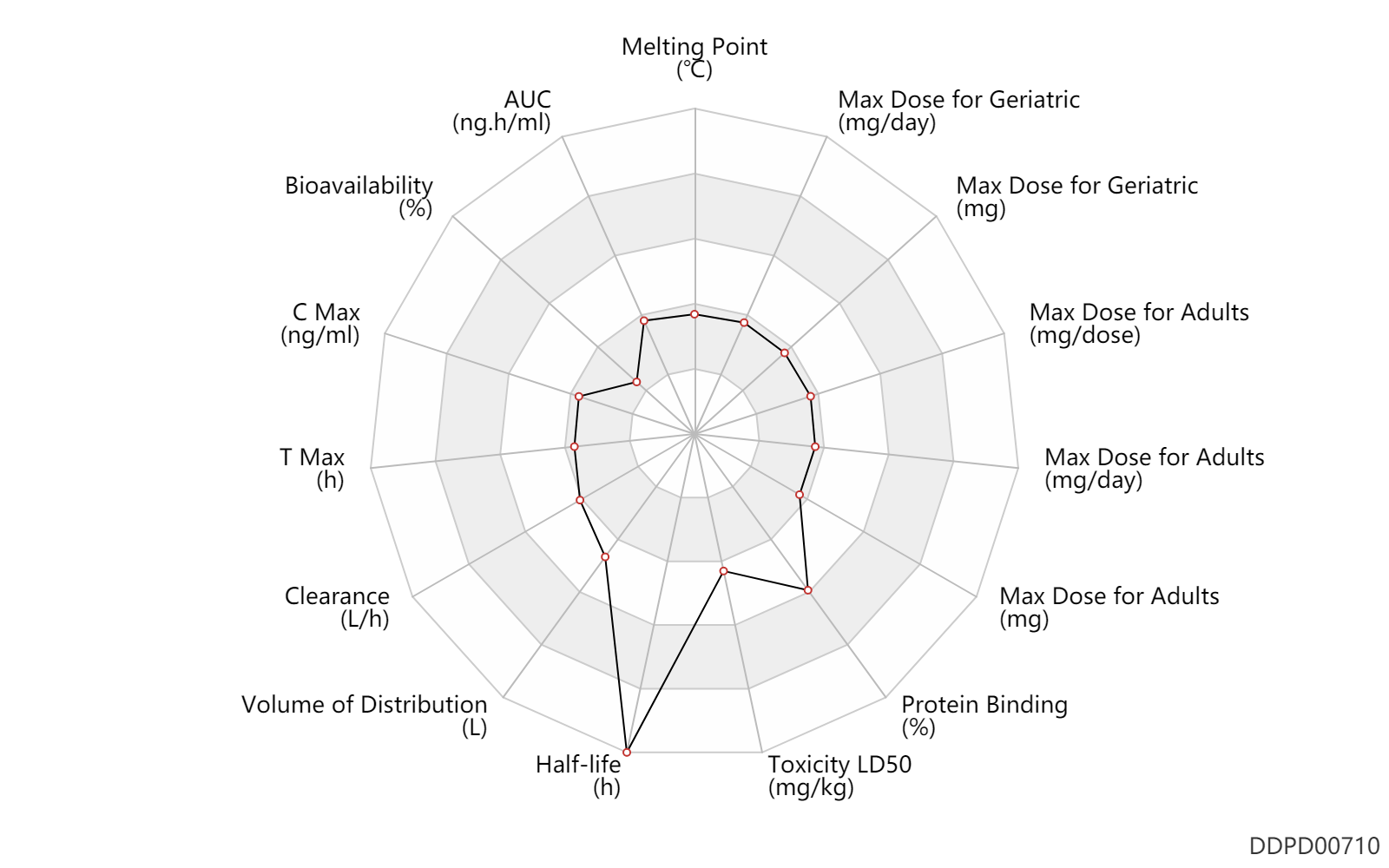
| Melting Point | 114.0 | ℃ | 113-115 | ℃ | ChemSpider |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| AUC | 316.0 | ng.h/ml | 316.0 | ng.h/ml | intravenous injection, IV; | DRUGBANK | AUC | 518.0 | ng.h/ml | 518.0 | ng.h/ml | intravenous injection, IV; | DRUGBANK | AUC | 908.0 | ng.h/ml | 908.0 | ng.h/ml | intravenous injection, IV; | DRUGBANK |
| Bioavailability | 0.63 | % | 0.63 | % | PO, oral; | DRUGBANK |
| C Max | 4.1 | ng/ml | 4.1±2.6 | ng/ml | PO, oral; normal,healthy; Male, men; | DRUGBANK |
| T Max | 1.1 | h | 1.1±0.6 | h | PO, oral; normal,healthy; Male, men; | DRUGBANK |
| Clearance | 7.3 | L/h | 84-160 | ml/min | DRUGBANK | |
| Volume of Distribution | 229.0 | L | 90-368 | L | Apparent volume of distribution; normal,healthy; | DRUGBANK | Volume of Distribution | 103.0 | L | 103.0 | L | Apparent volume of distribution; osteopenia; postmenopausal women; | DRUGBANK |
| Half-life | 97.0 | h | 37-157 | h | postmenopausal women; | DRUGBANK |
| Toxicity LD50 | 811.0 | mg/kg | 811.0 | mg/kg | PO, oral; Rattus, Rat; | T3DB |
| Protein Binding | 92.6 | % | 85.7-99.5 | % | DRUGBANK | Protein Binding | 86.0 | % | 86 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 2.5 | mg/day | 2.5 | mg/day | PO, oral | q3m | Boniva Tablets | ibandronate sodium | PDR |
| Max dose for adults | 5.0 | mg/day | 150 | mg/month | PO, oral | q3m | Boniva Tablets | ibandronate sodium | PDR |
| Max dose for adults | 3.0 | mg | 3 | mg | intravenous injection, IV | q3m | Boniva Tablets | ibandronate sodium | PDR |
| Max dose for adults | 50.0 | mg/day | 50 | mg/day | PO, oral | Boniva Tablets | ibandronate sodium | PDR | |
| Max dose for adults | 6.0 | mg/dose | 6 | mg/dose | intravenous infusion, iv in drop | Boniva Tablets | ibandronate sodium | PDR | |
| Max dose for geriatric | 2.5 | mg/day | 2.5 | mg/day | PO, oral | q3m | Boniva Tablets | ibandronate sodium | PDR |
| Max dose for geriatric | 5.0 | mg/day | 150 | mg/month | PO, oral | q3m | Boniva Tablets | ibandronate sodium | PDR |
| Max dose for geriatric | 3.0 | mg | 3 | mg | intravenous injection, IV | q3m | Boniva Tablets | ibandronate sodium | PDR |
| Max dose for geriatric | 50.0 | mg/day | 50 | mg/day | PO, oral | Boniva Tablets | ibandronate sodium | PDR | |
| Max dose for geriatric | 6.0 | mg/dose | 6 | mg/dose | intravenous infusion, iv in drop | Boniva Tablets | ibandronate sodium | PDR |