Basic Information
| Drug ID | DDPD00675 |

|
| Drug Name | Tamoxifen | |
| Molecular Weight | 371.5146 | |
| Molecular Formula | C26H29NO | |
| CAS Number | 10540-29-1 | |
| SMILES | CC\C(=C(/C1=CC=CC=C1)C1=CC=C(OCCN(C)C)C=C1)C1=CC=CC=C1 | |
| External Links | ||
| DRUGBANK | DB00675 | |
| T3DB | T3D4714 | |
| PubChem Compound | 2733526 | |
| PDR | 3809 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
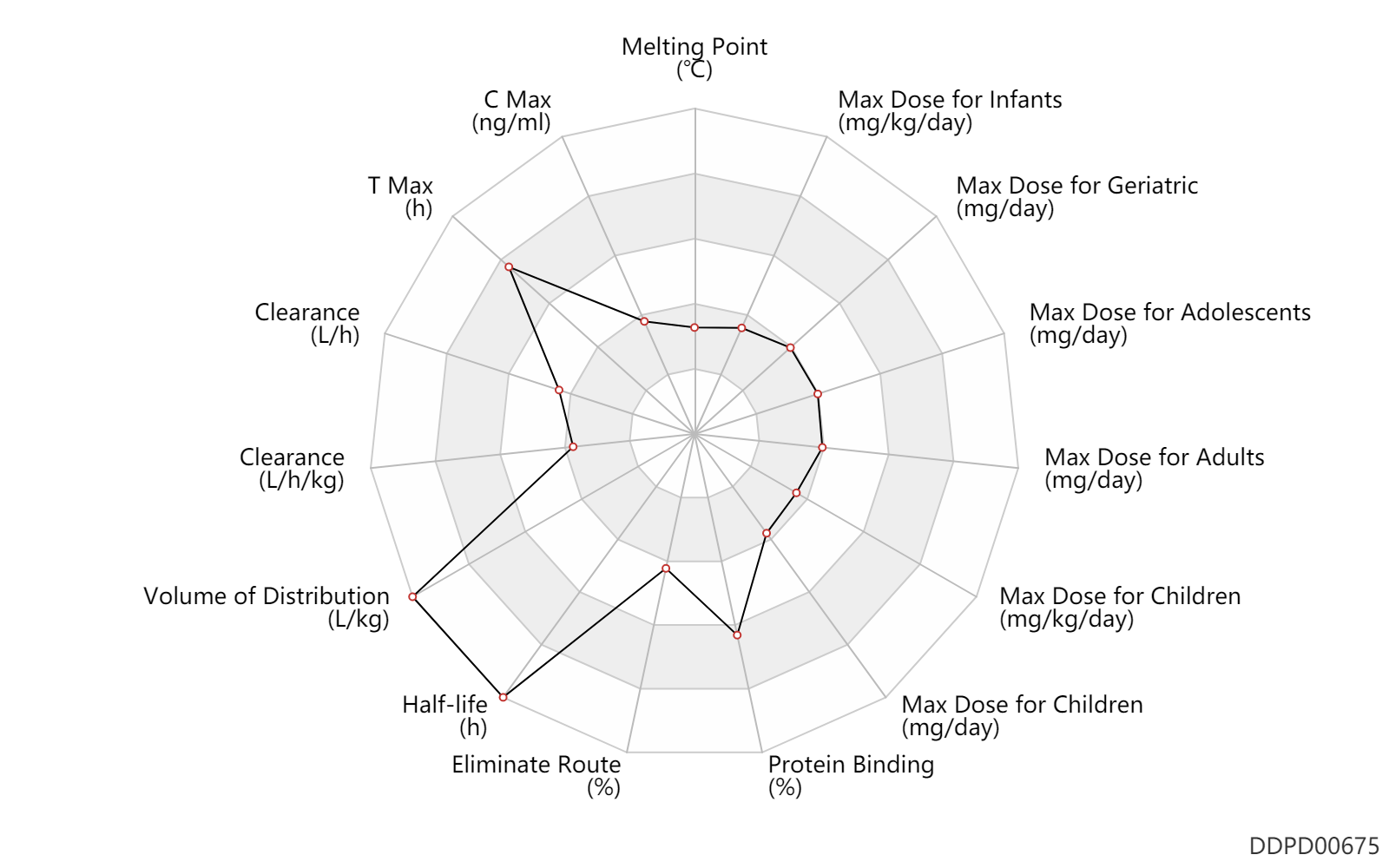
| Melting Point | 97.0 | ℃ | 97 | ℃ | http://www.chemspider.com/Chemical-Structure.2015313.html?rid=1b2fa2ba-dc6c-450e-bcf7-467741bd4eb1 |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| C Max | 40.0 | ng/ml | 40.0 | ng/ml | PO, oral; | DRUGBANK | C Max | 15.0 | ng/ml | 15.0 | ng/ml | PO, oral; Active metabolite; | DRUGBANK | C Max | 120.0 | ng/ml | 120(67-183) | ng/ml | PO, oral; | The Pharmacological Basis of Therapeutics |
| Css | 120.0 | ng/ml | 120.0 | ng/ml | PO, oral; | DRUGBANK | Css | 336.0 | ng/ml | 336.0 | ng/ml | PO, oral; different study; | DRUGBANK |
| T Max | 5.0 | h | 5 | h | PO, oral; | DRUGBANK | T Max | 5.0 | h | 5(3-7) | h | PO, oral; | The Pharmacological Basis of Therapeutics |
| Clearance | 11.3 | L/h | 189.0 | ml/min | postmenopausal women; | DRUGBANK | Clearance | 0.0840 | L/h/kg | 1.4 | ml/min/kg | apparent clearance; hydrolysis; | The Pharmacological Basis of Therapeutics |
| Volume of Distribution | 55.0 | L/kg | ~50-60 | L/kg | DRUGBANK | Volume of Distribution | 55.0 | L/kg | 50-60 | L/kg | Apparent volume of distribution; | The Pharmacological Basis of Therapeutics |
| Half-life | 144.0 | h | 5-7 | day | elimination half-life; | DRUGBANK | Half-life | 336.0 | h | ~14 | day | DRUGBANK | Half-life | 180.0 | h | 4-11 | day | at steady state; | The Pharmacological Basis of Therapeutics |
| Eliminate Route | 26.7 | % | 26.7 | % | Urinary excretion; human, homo sapiens; | DRUGBANK | Eliminate Route | 24.7 | % | 24.7 | % | Faeces excretion; human, homo sapiens; | DRUGBANK | Eliminate Route | 1.0 | % | <1 | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 98.0 | % | >98 | % | plasma proteins; | DRUGBANK | Protein Binding | 98.0 | % | >98 | % | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 1.0 | mg/kg/day | 1 | mg/kg/day | PO, oral | qd | Soltamox | tamoxifen citrate | PDR |
| Max dose for children | 1.0 | mg/kg/day | 1 | mg/kg/day | PO, oral | qd | Soltamox | tamoxifen citrate | PDR |
| Max dose for children | 20.0 | mg/day | 20 | mg/day | PO, oral | qd | Soltamox | tamoxifen citrate | PDR |
| Max dose for adolescents | 120.0 | mg/day | 120 | mg/day | PO, oral | qd | Soltamox | tamoxifen citrate | PDR |
| Max dose for adults | 20.0 | mg/day | 20 | mg/day | PO, oral | bid | Soltamox | tamoxifen citrate | PDR |
| Max dose for adults | 120.0 | mg/day | 120 | mg/day | PO, oral | Soltamox | tamoxifen citrate | PDR | |
| Max dose for adults | 80.0 | mg/m2 | 80 | mg/m2 | PO, oral | Soltamox | tamoxifen citrate | PDR | |
| Max dose for geriatric | 20.0 | mg/day | 20 | mg/day | PO, oral | bid | Soltamox | tamoxifen citrate | PDR |
| Max dose for geriatric | 120.0 | mg/day | 120 | mg/day | PO, oral | Soltamox | tamoxifen citrate | PDR | |
| Max dose for geriatric | 80.0 | mg/m2 | 80 | mg/m2 | PO, oral | Soltamox | tamoxifen citrate | PDR |