Basic Information
| Drug ID | DDPD00672 |

|
| Drug Name | Chlorpropamide | |
| Molecular Weight | 276.74 | |
| Molecular Formula | C10H13ClN2O3S | |
| CAS Number | 94-20-2 | |
| SMILES | CCCNC(=O)NS(=O)(=O)C1=CC=C(Cl)C=C1 | |
| External Links | ||
| DRUGBANK | DB00672 | |
| PubChem Compound | 2727 | |
| PDR | 3028 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
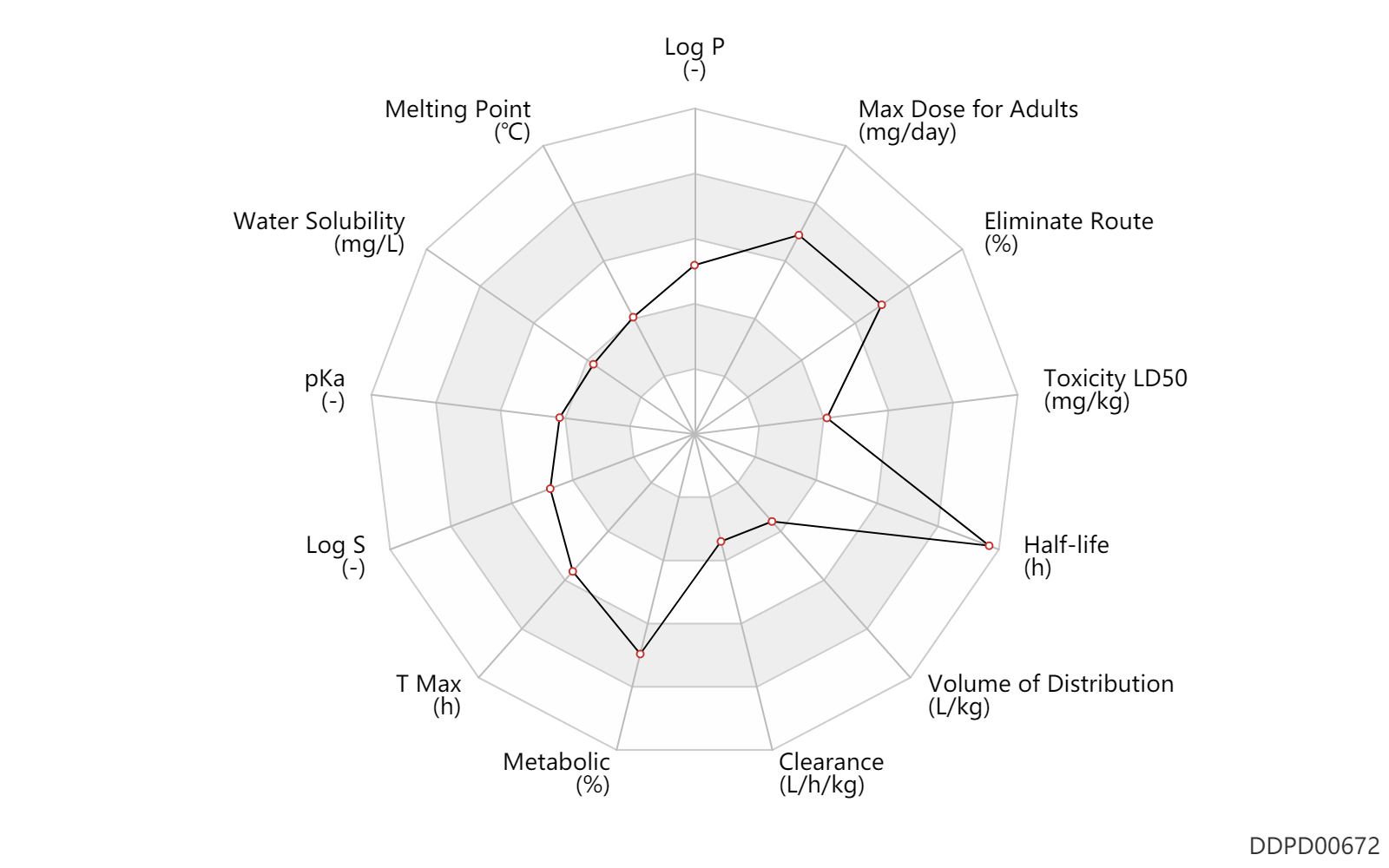
| Log P | 2.27 | - | 2.27 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 129.5 | ℃ | 129.2-129.8 | ℃ | McLamore, W.M.; U S . Patent 3,349,124; October 24,1967; assigned to Chas. Pfizer Co., Inc. |
| Water Solubility | 258.0 | mg/L | 258 | mg/L | YALKOWSKY,SH & DANNENFELSER,RM 1992) |
| pKa | 5.13 | - | 5.13 | - | LIPINSKI,CA ET AL. (1991) |
| Log S | -3.03 | - | -3.03 | - | ADME Research, USCD |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| T Max | 3.0 | h | 2-4 | h | DRUGBANK | |
| Metabolic | 80.0 | % | 80 | % | Liver metabolism; | DRUGBANK |
| Clearance | 0.002700 | L/h/kg | 0.045 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 0.19 | L/kg | 0.19 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 36.0 | h | ~36(25-60) | h | DRUGBANK | Half-life | 46.0 | h | 46 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 580.0 | mg/kg | 580.0 | mg/kg | Intraperitoneal, IP; Rattus, Rat; | DRUGBANK |
| Eliminate Route | 85.0 | % | 80-90 | % | Urinary excretion; Oral single dose; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 750.0 | mg/day | 750 | mg/day | PO, oral | Chlorpropamide | chlorpropamide | PDR |