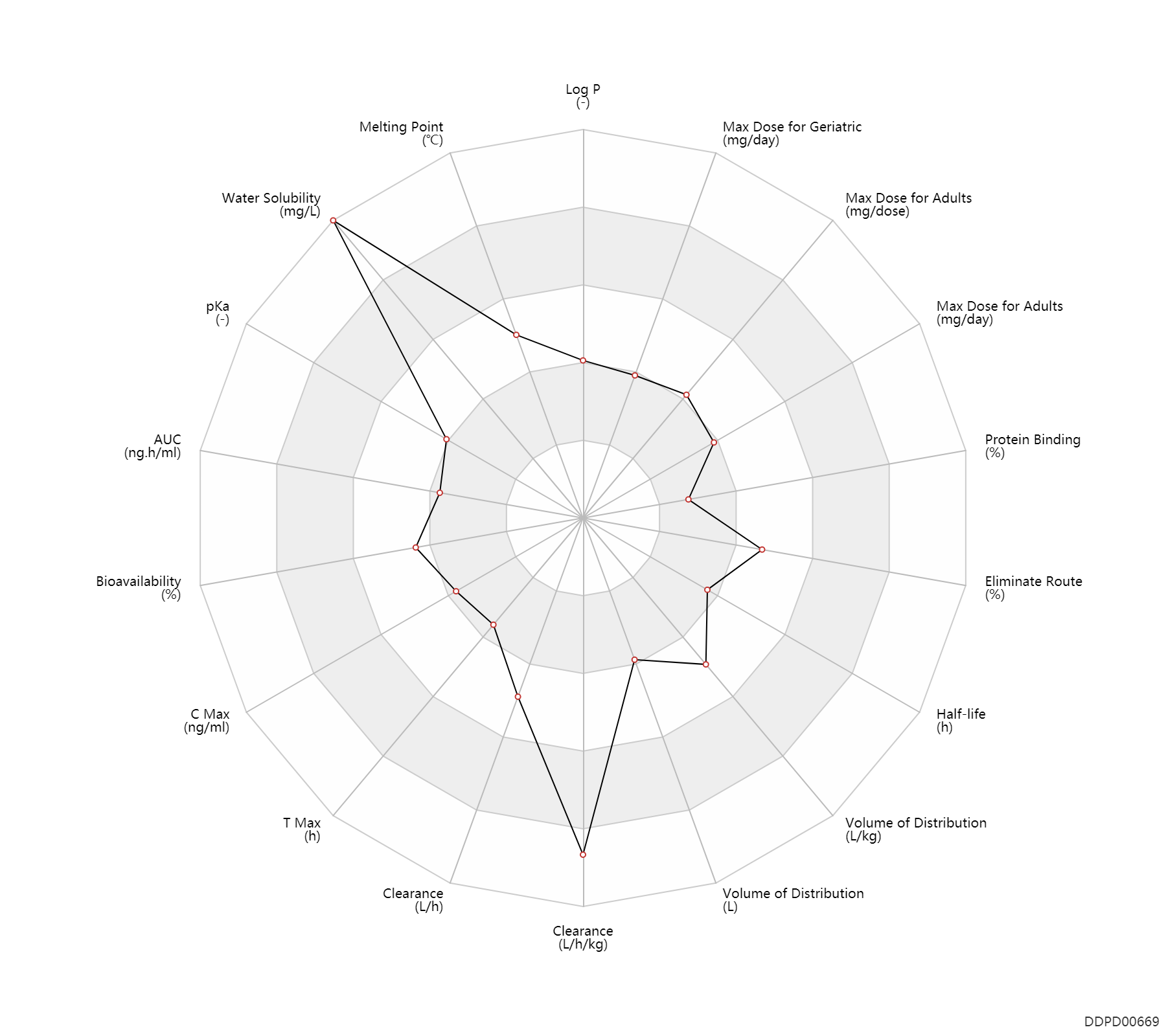
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Reference |
| AUC |
9.0 |
ng.h/ml |
9(7.5-10.9) |
ng.h/ml |
subcutaneous injection, SC; |
DRUGBANK |
AUC |
8.7 |
ng.h/ml |
8.7(6.1-12.5) |
ng.h/ml |
PO, oral; |
DRUGBANK |
AUC |
7.4 |
ng.h/ml |
7.4(5-10.8) |
ng.h/ml |
inhalation, IH; |
DRUGBANK |
AUC |
14.6 |
ng.h/ml |
14.6(11.3-18.8) |
ng.h/ml |
Rectal Administration; |
DRUGBANK |
| Bioavailability |
100.0 |
% |
100 |
% |
subcutaneous injection, SC; |
DRUGBANK |
Bioavailability |
14.3 |
% |
14.3(11.4-17.9) |
% |
PO, oral; |
DRUGBANK |
Bioavailability |
15.8 |
% |
15.8(12.6-19.8) |
% |
inhalation, IH; |
DRUGBANK |
Bioavailability |
19.2 |
% |
19.2(15.3-24.1) |
% |
Rectal Administration; |
DRUGBANK |
Bioavailability |
14.0 |
% |
14±5 |
% |
PO, oral; |
The Pharmacological Basis of Therapeutics |
Bioavailability |
97.0 |
% |
97±16 |
% |
subcutaneous injection, SC; |
The Pharmacological Basis of Therapeutics |
| C Max |
69.5 |
ng/ml |
69.5(62.8-76.9) |
ng/ml |
subcutaneous injection, SC; |
DRUGBANK |
C Max |
16.5 |
ng/ml |
16.5(13.5-20.1) |
ng/ml |
PO, oral; |
DRUGBANK |
C Max |
12.9 |
ng/ml |
12.9(10.5-15.9) |
ng/ml |
inhalation, IH; |
DRUGBANK |
C Max |
22.9 |
ng/ml |
22.9(18.4-28.6) |
ng/ml |
Rectal Administration; |
DRUGBANK |
C Max |
54.0 |
ng/ml |
54(27-137) |
ng/ml |
Oral single dose; adults; normal,healthy; |
The Pharmacological Basis of Therapeutics |
C Max |
72.0 |
ng/ml |
72(55-108) |
ng/ml |
adults; normal,healthy; |
The Pharmacological Basis of Therapeutics |
| T Max |
0.17 |
h |
0.17(0.08-0.33) |
h |
subcutaneous injection, SC; |
DRUGBANK |
T Max |
1.5 |
h |
1.5(0.5-2) |
h |
PO, oral; |
DRUGBANK |
T Max |
1.5 |
h |
1.5(0.25-3) |
h |
inhalation, IH; |
DRUGBANK |
T Max |
1.0 |
h |
1(0.75-3) |
h |
Rectal Administration; |
DRUGBANK |
T Max |
1.5 |
h |
~1.5 |
h |
Oral single dose; adults; normal,healthy; |
The Pharmacological Basis of Therapeutics |
T Max |
0.20 |
h |
0.2(0.1-0.3) |
h |
adults; normal,healthy; |
The Pharmacological Basis of Therapeutics |
| Clearance |
0.0132 |
L/h |
0.22(0.19-0.25) |
ml/min |
subcutaneous injection, SC; |
DRUGBANK |
Clearance |
0.0105 |
L/h |
0.14-0.21 |
ml/min |
PO, oral; Rectal Administration; |
DRUGBANK |
Clearance |
0.0129 |
L/h |
0.18-0.25 |
ml/min |
IM,intramuscular injection; |
DRUGBANK |
Clearance |
72.0 |
L/h |
~1200 |
ml/min |
Plasma clearance; |
DRUGBANK |
Clearance |
1.3 |
L/h/kg |
22±5.4 |
ml/min/kg |
normal,healthy; adults; |
The Pharmacological Basis of Therapeutics |
Clearance |
1.1 |
L/h/kg |
19 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
50.0 |
L |
50±8 |
L |
subcutaneous injection, SC; |
DRUGBANK |
Volume of Distribution |
2.0 |
L/kg |
2.0±0.34 |
L/kg |
normal,healthy; adults; |
The Pharmacological Basis of Therapeutics |
Volume of Distribution |
1.7 |
L/kg |
1.7 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
1.9 |
h |
1.9 |
h |
subcutaneous injection, SC; |
DRUGBANK |
Half-life |
1.9 |
h |
1.7-2.0 |
h |
subcutaneous injection, SC; 95%CI; |
DRUGBANK |
Half-life |
1.7 |
h |
1.7 |
h |
PO, oral; |
DRUGBANK |
Half-life |
1.7 |
h |
1.4-1.9 |
h |
PO, oral; 95%CI; |
DRUGBANK |
Half-life |
1.8 |
h |
1.8 |
h |
Rectal Administration; |
DRUGBANK |
Half-life |
1.9 |
h |
1.6-2.2 |
h |
Rectal Administration; 95%CI; |
DRUGBANK |
Half-life |
1.8 |
h |
1.8 |
h |
|
DRUGBANK |
Half-life |
1.9 |
h |
1.7-2.0 |
h |
95%CI; |
DRUGBANK |
Half-life |
1.0 |
h |
1.0±0.3 |
h |
|
The Pharmacological Basis of Therapeutics |
Half-life |
2.0 |
h |
~2 |
h |
subcutaneous injection, SC; PO, oral; |
The Pharmacological Basis of Therapeutics |
Half-life |
1.7 |
h |
1.7 |
h |
intravenous injection, IV; human, homo sapiens; |
Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route |
40.0 |
% |
~40 |
% |
Faeces excretion; |
DRUGBANK |
Eliminate Route |
24.0 |
% |
24±4 |
% |
Urinary excretion; Unchanged drug; |
DRUGBANK |
Eliminate Route |
22.0 |
% |
22±4 |
% |
Urinary excretion; Unchanged drug; |
The Pharmacological Basis of Therapeutics |
| Protein Binding |
17.5 |
% |
14-21 |
% |
|
DRUGBANK |
Protein Binding |
17.5 |
% |
14-21 |
% |
|
The Pharmacological Basis of Therapeutics |