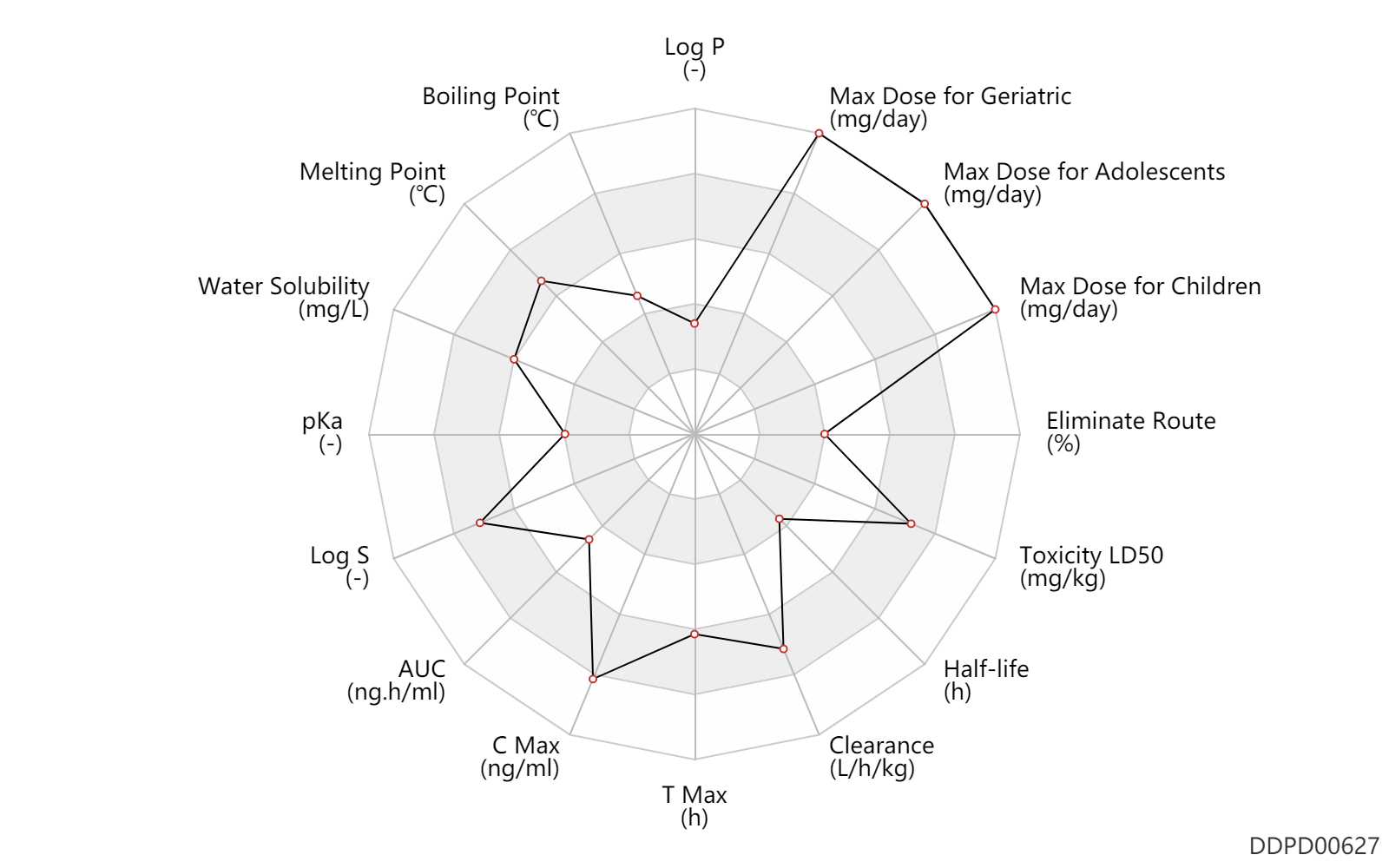
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Reference |
| AUC |
1440.0 |
ng.h/ml |
1.44 |
ug.h/ml |
PO, oral; RD, renal impairment, Renal disease,including uremia; |
DRUGBANK |
AUC |
6660.0 |
ng.h/ml |
6.66 |
ug.h/ml |
PO, oral; RD, renal impairment, Renal disease,including uremia; |
DRUGBANK |
AUC |
12410.0 |
ng.h/ml |
12.41 |
ug.h/ml |
PO, oral; RD, renal impairment, Renal disease,including uremia; |
DRUGBANK |
| C Max |
60.0 |
ng/ml |
0.06 |
ug/ml |
PO, oral; RD, renal impairment, Renal disease,including uremia; |
DRUGBANK |
C Max |
2420.0 |
ng/ml |
2.42 |
ug/ml |
PO, oral; RD, renal impairment, Renal disease,including uremia; |
DRUGBANK |
C Max |
4220.0 |
ng/ml |
4.22 |
ug/ml |
PO, oral; RD, renal impairment, Renal disease,including uremia; |
DRUGBANK |
C Max |
600.0 |
ng/ml |
0.6 |
mcg/ml |
PO, oral; extended release formulation; |
The Pharmacological Basis of Therapeutics |
C Max |
15500.0 |
ng/ml |
15.5 |
mcg/ml |
PO, oral; extended release formulation; |
The Pharmacological Basis of Therapeutics |
| T Max |
3.0 |
h |
3 |
h |
PO, oral; RD, renal impairment, Renal disease,including uremia; |
DRUGBANK |
T Max |
3.0 |
h |
3 |
h |
PO, oral; RD, renal impairment, Renal disease,including uremia; |
DRUGBANK |
T Max |
4.5 |
h |
4-5 |
h |
PO, oral; extended release formulation; |
The Pharmacological Basis of Therapeutics |
| Clearance |
0.88 |
L/h/kg |
14.6±5.0 |
ml/min/kg |
|
The Pharmacological Basis of Therapeutics |
| Half-life |
0.90 |
h |
0.9 |
h |
|
DRUGBANK |
Half-life |
1.3 |
h |
1.3 |
h |
|
DRUGBANK |
Half-life |
4.3 |
h |
4.3 |
h |
|
DRUGBANK |
Half-life |
0.20 |
h |
~0.15-0.25 |
h |
intravenous infusion, IV in drop; |
The Pharmacological Basis of Therapeutics |
| Toxicity LD50 |
3720.0 |
mg/kg |
3720.0 |
mg/kg |
PO, oral; mouse; |
DRUGBANK |
Toxicity LD50 |
4550.0 |
mg/kg |
4550.0 |
mg/kg |
PO, oral; rabbit; |
DRUGBANK |
Toxicity LD50 |
7000.0 |
mg/kg |
7000.0 |
mg/kg |
PO, oral; Rattus, Rat; |
DRUGBANK |
Toxicity LD50 |
2000.0 |
mg/kg |
2000.0 |
mg/kg |
skin/dermal; Rattus, Rat; |
DRUGBANK |
Toxicity LD50 |
7000.0 |
mg/kg |
7000.0 |
mg/kg |
PO, oral; Rattus, Rat; |
T3DB |
Toxicity LD50 |
730.0 |
mg/kg |
730.0 |
mg/kg |
Intraperitoneal, IP; Rattus, Rat; |
T3DB |
Toxicity LD50 |
3500.0 |
mg/kg |
3500.0 |
mg/kg |
subcutaneous injection, SC; mouse; |
T3DB |
| Eliminate Route |
12.0 |
% |
12 |
% |
Urinary excretion; Oral multiple dose; Unchanged drug; |
The Pharmacological Basis of Therapeutics |