Basic Information
| Drug ID | DDPD00625 |

|
| Drug Name | Efavirenz | |
| Molecular Weight | 315.675 | |
| Molecular Formula | C14H9ClF3NO2 | |
| CAS Number | 154598-52-4 | |
| SMILES | FC(F)(F)[C@]1(OC(=O)NC2=C1C=C(Cl)C=C2)C#CC1CC1 | |
| External Links | ||
| DRUGBANK | DB00625 | |
| PubChem Compound | 64139 | |
| PDR | 116 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
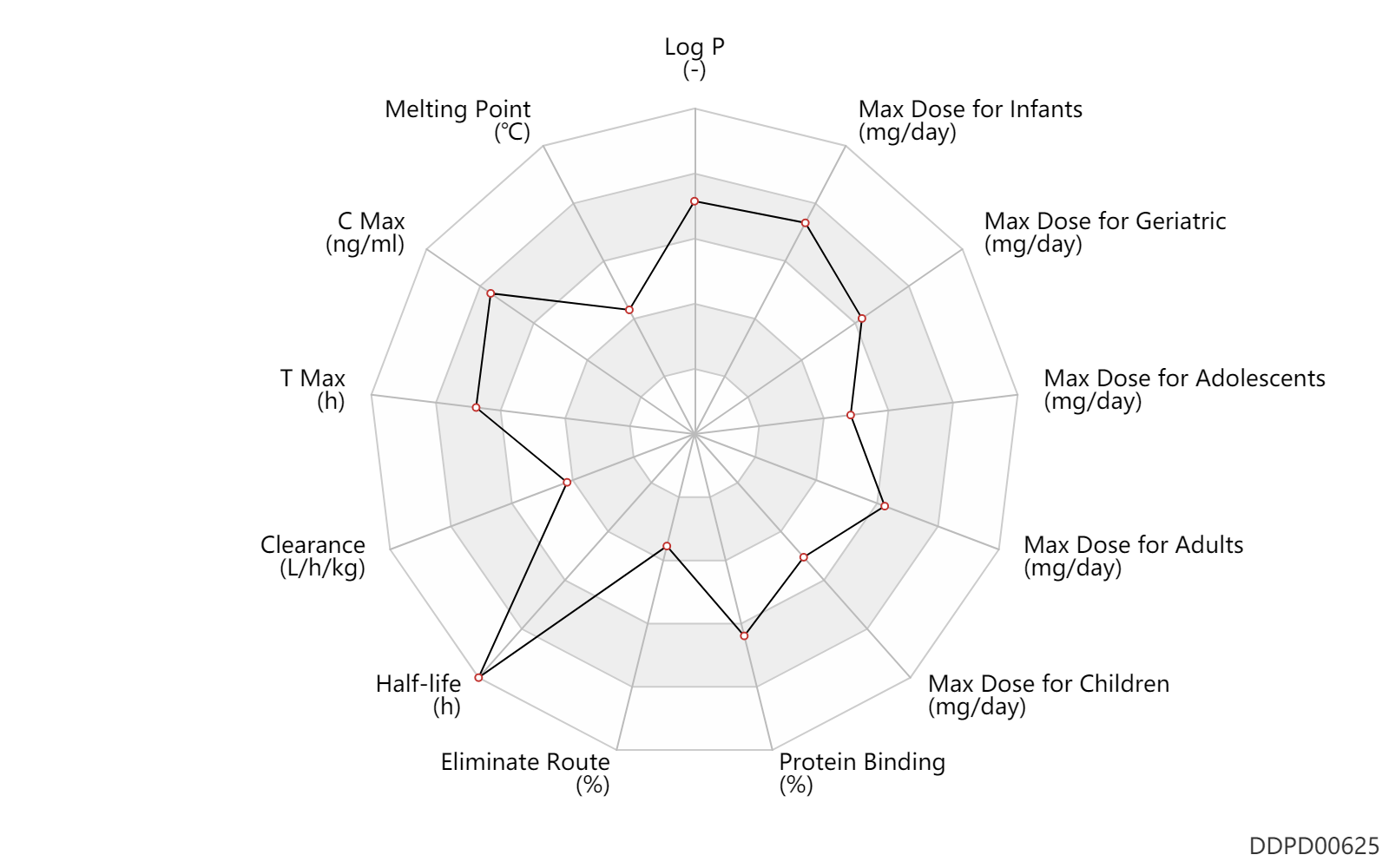
| Log P | 4.6 | - | 4.6 | - | DRUGBANK |
| Melting Point | 140.0 | ℃ | 139-141 | ℃ | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| C Max | 4000.0 | ng/ml | 4.0±1.7 | mcg/ml | PO, oral; | The Pharmacological Basis of Therapeutics | |
| T Max | 4.1 | h | 4.1±1.7 | h | PO, oral; | The Pharmacological Basis of Therapeutics | |
| Clearance | 0.19 | L/h/kg | 3.1±1.2 | ml/min/kg | apparent clearance; PO, oral; | Children → ; | The Pharmacological Basis of Therapeutics |
| Half-life | 47.5 | h | 40-55 | h | DRUGBANK | Half-life | 64.0 | h | 52-76 | h | Oral single dose; | The Pharmacological Basis of Therapeutics | Half-life | 47.5 | h | 40-55 | h | Oral multiple dose; | The Pharmacological Basis of Therapeutics |
| Eliminate Route | 1.0 | % | <1 | % | Urinary excretion; AIDS,HIV; human, homo sapiens; Unchanged drug; | The Pharmacological Basis of Therapeutics | |
| Protein Binding | 99.6 | % | 99.5-99.75 | % | DRUGBANK | Protein Binding | 99.6 | % | 99.5-99.75 | % | AIDS,HIV; | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 200.0 | mg/day | 200 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for infants | 150.0 | mg/day | 150 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for infants | 100.0 | mg/day | 100 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for children | 600.0 | mg/day | 600 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for children | 400.0 | mg/day | 400 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for children | 350.0 | mg/day | 350 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for children | 300.0 | mg/day | 300 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for children | 250.0 | mg/day | 250 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for children | 200.0 | mg/day | 200 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for children | 150.0 | mg/day | 150 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for adolescents | 600.0 | mg/day | 600 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for adolescents | 400.0 | mg/day | 400 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for adolescents | 350.0 | mg/day | 350 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for adults | 600.0 | mg/day | 600 | mg/day | PO, oral | Sustiva | efavirenz | PDR |
| Max dose for geriatric | 600.0 | mg/day | 600 | mg/day | PO, oral | Sustiva | efavirenz | PDR |