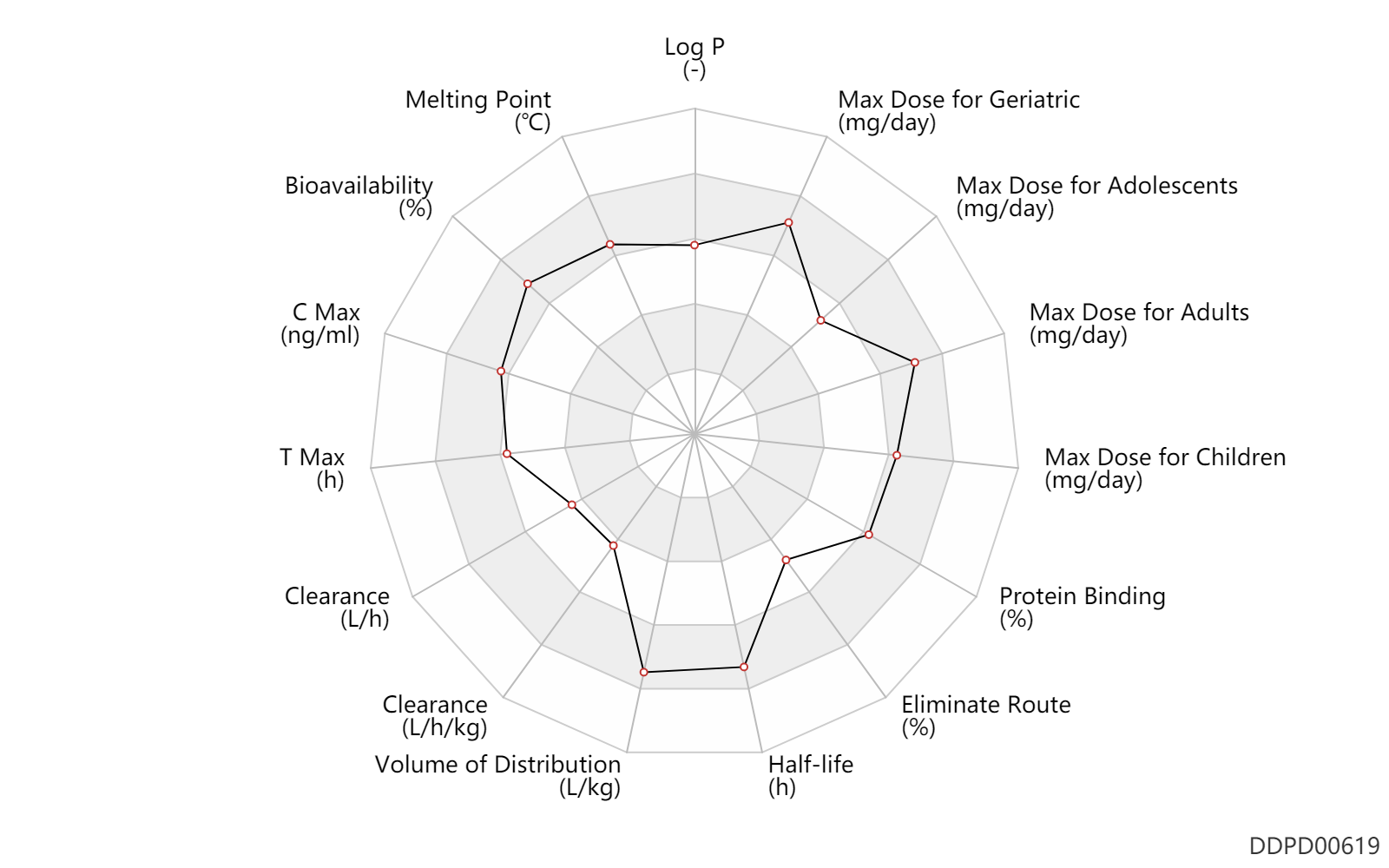
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| Bioavailability |
98.0 |
% |
98 |
% |
|
|
DRUGBANK |
Bioavailability |
98.0 |
% |
98(87-111) |
% |
PO, oral; |
|
The Pharmacological Basis of Therapeutics |
| C Max |
2600.0 |
ng/ml |
2.6±0.8 |
mcg/ml |
PO, oral; |
|
The Pharmacological Basis of Therapeutics |
| T Max |
3.0 |
h |
2-4 |
h |
|
|
DRUGBANK |
T Max |
3.3 |
h |
3.3±1.1 |
h |
PO, oral; |
|
The Pharmacological Basis of Therapeutics |
| Clearance |
11.0 |
L/h |
8.0-14 |
L/h |
Gastrointestinal stromal tumor; Chronic myelogenous leukemia; patients; |
gaining weight ↑ ; |
DRUGBANK |
Clearance |
0.20 |
L/h/kg |
3.3±1.2 |
ml/min/kg |
|
|
The Pharmacological Basis of Therapeutics |
Clearance |
0.20 |
L/h/kg |
3.3 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
6.2 |
L/kg |
6.2±2.2 |
L/kg |
|
|
The Pharmacological Basis of Therapeutics |
Volume of Distribution |
3.9 |
L/kg |
3.9 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
18.0 |
h |
18 |
h |
normal,healthy; |
|
DRUGBANK |
Half-life |
40.0 |
h |
40 |
h |
normal,healthy; |
|
DRUGBANK |
Half-life |
22.0 |
h |
22±4 |
h |
|
|
The Pharmacological Basis of Therapeutics |
Half-life |
22.0 |
h |
22 |
h |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route |
68.0 |
% |
68 |
% |
Faeces excretion; |
|
DRUGBANK |
Eliminate Route |
13.0 |
% |
13 |
% |
Urinary excretion; |
|
DRUGBANK |
Eliminate Route |
5.0 |
% |
5 |
% |
Urinary excretion; Unchanged drug; |
|
DRUGBANK |
Eliminate Route |
20.0 |
% |
20 |
% |
Faeces excretion; Unchanged drug; |
|
DRUGBANK |
Eliminate Route |
50.0 |
% |
5 |
% |
Urinary excretion; Unchanged drug; |
|
The Pharmacological Basis of Therapeutics |
| Protein Binding |
95.0 |
% |
95 |
% |
|
|
DRUGBANK |
Protein Binding |
95.0 |
% |
95 |
% |
|
|
The Pharmacological Basis of Therapeutics |