Basic Information
| Drug ID | DDPD00602 |

|
| Drug Name | Ivermectin | |
| Molecular Weight | 1736.1589 | |
| Molecular Formula | C95H146O28 | |
| CAS Number | 70288-86-7 | |
| SMILES | CO[C@H]1C[C@@H](O[C@@H](C)[C@@H]1O)O[C@H]1[C@H](C)O[C@H](C[C@@H]1OC)O[C@H]1[C@@H](C)\C=C\C=C2/CO[C@@H]3[C@H](O)C(C)=C[C@@H](C(=O)O[C@H]4C[C@@H](C\C=C1/C)O[C@@]1(CC[C@H](C)[C@@H](C(C)C)O1)C4)[C@]23O.CC[C@@H](C)[C@H]1O[C@@]2(CC[C@@H]1C)O[C@@H]1C\C=C(C)\[C@@H](O[C@@H]3O[C@@H](C)[C@H](O[C@@H]4O[C@@H](C)[C@H](O)[C@@H](OC)C4)[C@@H](OC)C3)[C@@H](C)\C=C\C=C3/CO[C@@H]4[C@H](O)C(C)=C[C@@H](C(=O)O[C@@H](C1)C2)[C@]34O | |
| External Links | ||
| DRUGBANK | DB00602 | |
| T3DB | T3D4996 | |
| PubChem Compound | 46936176 | |
| PDR | 2415 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
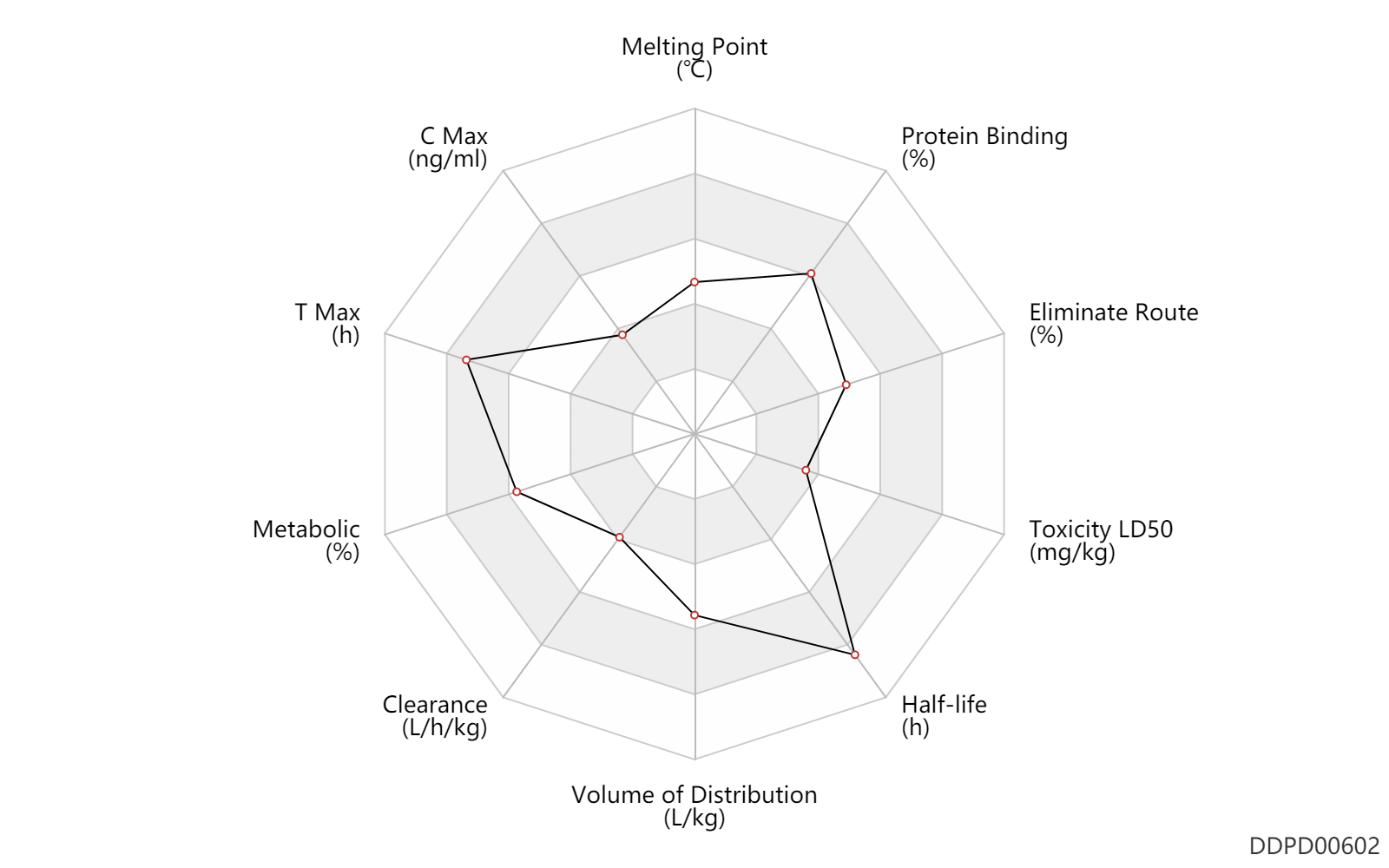
| Melting Point | 155.0 | ℃ | 155 | ℃ | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| C Max | 38.2 | ng/ml | 38.2±5.8 | ng/ml | onchocerciasis; | The Pharmacological Basis of Therapeutics |
| T Max | 4.7 | h | 4.7±0.5 | h | onchocerciasis; | The Pharmacological Basis of Therapeutics |
| Metabolic | 99.0 | % | 99 | % | Faeces excretion; | DRUGBANK | Metabolic | 1.0 | % | <1 | % | Urinary excretion; | DRUGBANK |
| Clearance | 0.12 | L/h/kg | 2.06±0.81 | ml/min/kg | apparent clearance; | The Pharmacological Basis of Therapeutics |
| Volume of Distribution | 3.3 | L/kg | 3-3.5 | L/kg | DRUGBANK | Volume of Distribution | 2.1 | L/kg | 2.06±0.81 | L/kg | Apparent volume of distribution; | The Pharmacological Basis of Therapeutics |
| Half-life | 16.0 | h | 16 | h | DRUGBANK | Half-life | 25.0 | h | 22-28 | h | different study; | DRUGBANK | Half-life | 56.5 | h | 56.5±7.5 | h | terminal half-life; Male, men; Female, women; patients; onchocerciasis; | The Pharmacological Basis of Therapeutics |
| Toxicity LD50 | 29.5 | mg/kg | 29.5 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 10.0 | mg/kg | 10.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 29.5 | mg/kg | 29.5 | mg/kg | PO, oral; mouse; | T3DB | Toxicity LD50 | 10.0 | mg/kg | 10.0 | mg/kg | PO, oral; Rattus, Rat; | T3DB | Toxicity LD50 | 29.5 | mg/kg | 29.5 | mg/kg | PO, oral; mouse; | T3DB | Toxicity LD50 | 10.0 | mg/kg | 10.0 | mg/kg | PO, oral; Rattus, Rat; | T3DB |
| Eliminate Route | 100.0 | % | ~100 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 1.0 | % | <1 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 1.0 | % | <1 | % | Urinary excretion; onchocerciasis; human, homo sapiens; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 93.0 | % | 93 | % | DRUGBANK | Protein Binding | 93.1 | % | 93.1±0.2 | % | onchocerciasis; human, homo sapiens; | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 4.0 | oz/topicaL appLication | 4 | oz/topicaL appLication | skin/dermal | Sklice | ivermectin | PDR |
| Max dose for children | 4.0 | oz/topical application | 4 | oz/topical application | skin/dermal | Sklice | ivermectin | PDR |
| Max dose for children | 4.0 | oz/topical application | 4 | oz/topical application | skin/dermal | Sklice | ivermectin | PDR |
| Max dose for children | 0.175 | mg/kg | 175 | mcg/kg | PO, oral | Sklice | ivermectin | PDR |
| Max dose for adolescents | 4.0 | oz/topical application | 4 | oz/topical application | skin/dermal | Sklice | ivermectin | PDR |
| Max dose for adolescents | 0.175 | mg/kg/dose | 175 | mcg/kg/dose | PO, oral | Sklice | ivermectin | PDR |
| Max dose for adults | 0.4 | mg/kg | 400 | mcg/kg | PO, oral | Sklice | ivermectin | PDR |
| Max dose for adults | 4.0 | oz/topicaL appLication | 4 | oz/topicaL appLication | skin/dermal | Sklice | ivermectin | PDR |
| Max dose for adults | 175.0 | mcg/kg/dose | 175 | mcg/kg/dose | PO, oral | Sklice | ivermectin | PDR |
| Max dose for geriatric | 4.0 | oz/topical application | 4 | oz/topical application | skin/dermal | Sklice | ivermectin | PDR |
| Max dose for geriatric | 175.0 | mcg/kg/dose | 175 | mcg/kg/dose | PO, oral | Sklice | ivermectin | PDR |