Basic Information
| Drug ID | DDPD00594 |

|
| Drug Name | Amiloride | |
| Molecular Weight | 229.627 | |
| Molecular Formula | C6H8ClN7O | |
| CAS Number | 2609-46-3 | |
| SMILES | NC(=N)NC(=O)C1=NC(Cl)=C(N)N=C1N | |
| External Links | ||
| DRUGBANK | DB00594 | |
| PubChem Compound | 16231 | |
| PDR | 1838 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
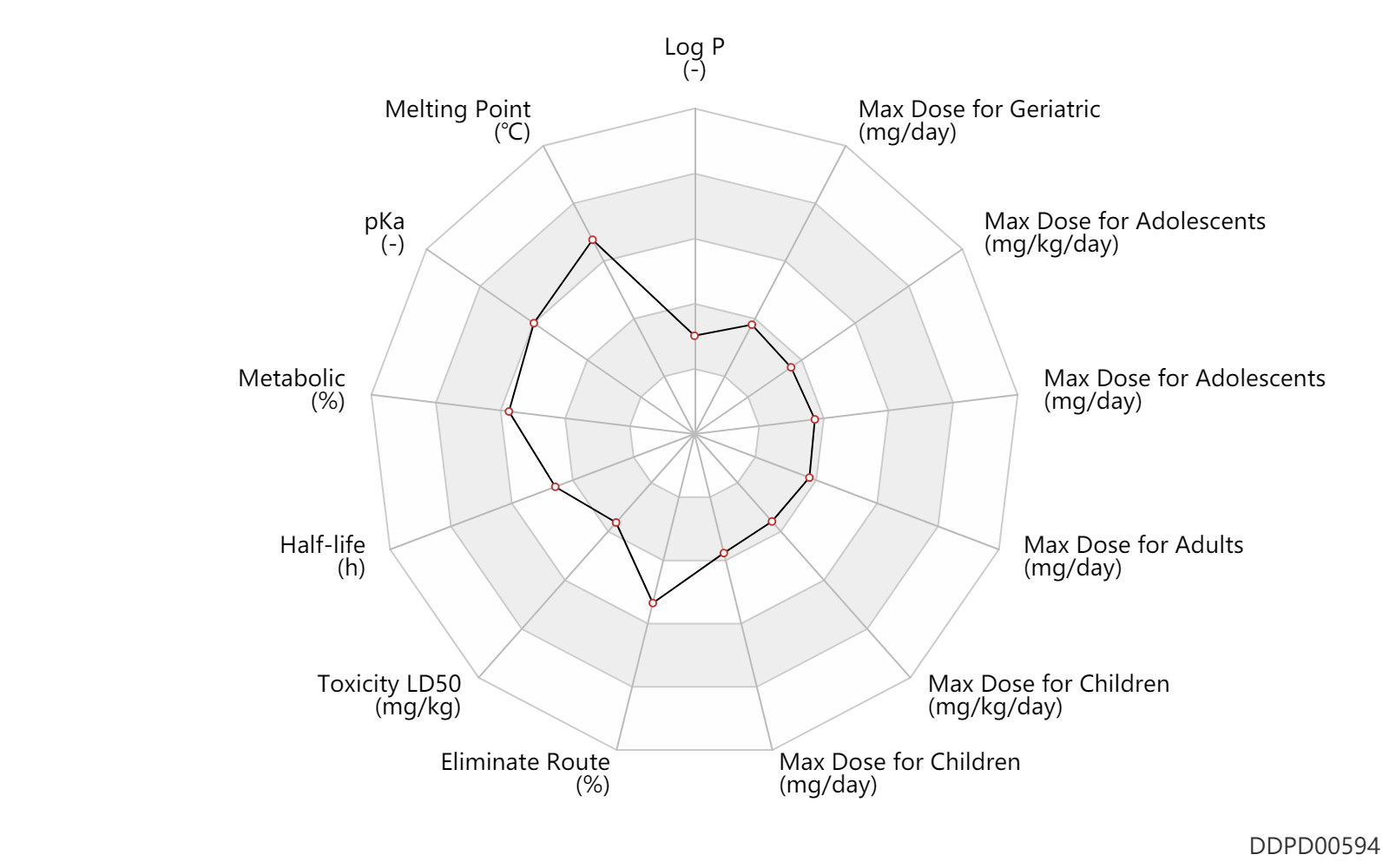
| Log P | -0.3 | - | -0.3 | - | DRUGBANK |
| Melting Point | 241.0 | ℃ | 240.5-241.5 | ℃ | Cragoe, E.J., Jr.; US. Patent 3,313,813; April 11,1967; assigned to Merck 81 Co., Inc. |
| pKa | 8.7 | - | 8.7 | - | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Metabolic | 0 | % | 0 | % | Liver metabolism; | DRUGBANK | Metabolic | 100.0 | % | 100 | % | Urinary excretion; | DRUGBANK |
| Half-life | 7.5 | h | 6-9 | h | elimination half-life; | DRUGBANK |
| Toxicity LD50 | 56.0 | mg/kg | 56.0 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 60.5 | mg/kg | 36-85 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK |
| Eliminate Route | 50.0 | % | ~50 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 40.0 | % | ~40 | % | Faeces excretion; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 0.625 | mg/kg/day | 0.625 | mg/kg/day | PO, oral | Midamor | amiloride hydrochloride | PDR |
| Max dose for children | 20.0 | mg/day | 20 | mg/day | PO, oral | Midamor | amiloride hydrochloride | PDR |
| Max dose for adolescents | 0.625 | mg/kg/day | 0.625 | mg/kg/day | PO, oral | Midamor | amiloride hydrochloride | PDR |
| Max dose for adolescents | 20.0 | mg/day | 20 | mg/day | PO, oral | Midamor | amiloride hydrochloride | PDR |
| Max dose for adults | 20.0 | mg/day | 20 | mg/day | PO, oral | Midamor | amiloride hydrochloride | PDR |
| Max dose for adults | 40.0 | mg/day | 40 | mg/day | PO, oral | Midamor | amiloride hydrochloride | PDR |
| Max dose for geriatric | 20.0 | mg/day | 20 | mg/day | PO, oral | Midamor | amiloride hydrochloride | PDR |
| Max dose for geriatric | 40.0 | mg/day | 40 | mg/day | PO, oral | Midamor | amiloride hydrochloride | PDR |