Basic Information
| Drug ID | DDPD00591 |

|
| Drug Name | Fluocinolone acetonide | |
| Molecular Weight | 452.4882 | |
| Molecular Formula | C24H30F2O6 | |
| CAS Number | 67-73-2 | |
| SMILES | [H][C@@]12C[C@@]3([H])[C@]4([H])C[C@H](F)C5=CC(=O)C=C[C@]5(C)[C@@]4(F)[C@@H](O)C[C@]3(C)[C@@]1(OC(C)(C)O2)C(=O)CO | |
| External Links | ||
| DRUGBANK | DB00591 | |
| PubChem Compound | 6215 | |
| PDR | 1674 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
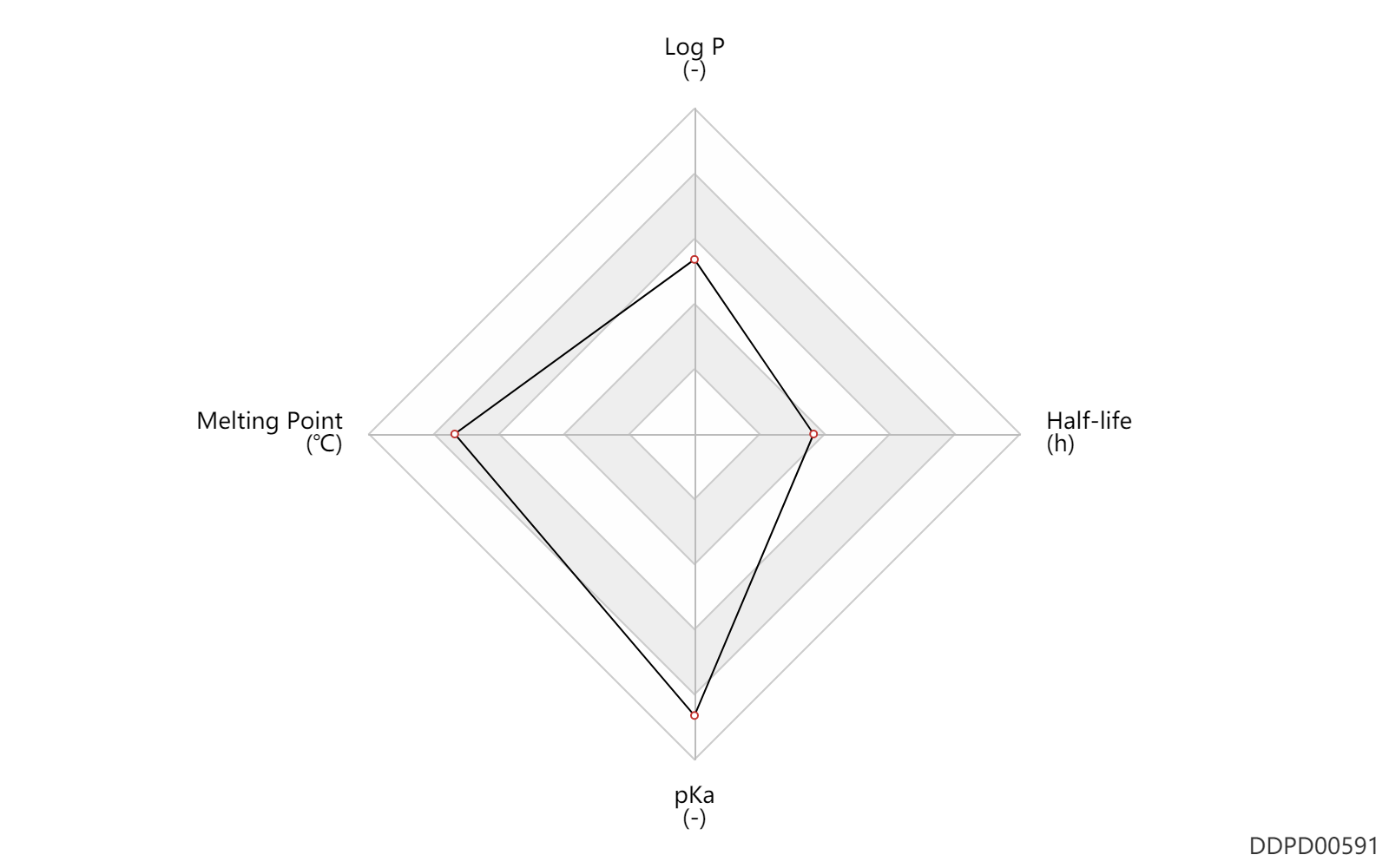
| Log P | 2.48 | - | 2.48 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 267.0 | ℃ | 266-268 | ℃ | Mills, J.S. and Bowers, A.; U.S. Patent 3,014,938; December 26, 1961; assigned to Syntex SA, Mexico. |
| pKa | 13.9 | - | 13.9 | - | Graham P. (2013). An Introduction to Medicinal Chemistry. Fifth ed. Oxford. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Half-life | 1.5 | h | 1.3-1.7 | h | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 4.0 | appLication/day | 4 | appLication/day | Retisert | fluocinolone acetonide | PDR | |
| Max dose for infants | 2.0 | appLication/day | 2 | appLication/day | Retisert | fluocinolone acetonide | PDR | |
| Max dose for infants | 2.0 | appLication/day | 2 | appLication/day | Retisert | fluocinolone acetonide | PDR | |
| Max dose for children | 4.0 | application/day | 4 | application/day | Retisert | fluocinolone acetonide | PDR | |
| Max dose for children | 2.0 | application/day | 2 | application/day | Retisert | fluocinolone acetonide | PDR | |
| Max dose for adolescents | 4.0 | application/day | 4 | application/day | skin/dermal | Retisert | fluocinolone acetonide | PDR |
| Max dose for adolescents | 3.0 | application/day | 3 | application/day | skin/dermal | Retisert | fluocinolone acetonide | PDR |
| Max dose for adolescents | 30.0 | ml/day | 30 | ml/day | skin/dermal | Retisert | fluocinolone acetonide | PDR |
| Max dose for adolescents | 1.0 | implant/30 months | 1 | implant/30 months | subdermal implant | Retisert | fluocinolone acetonide | PDR |
| Max dose for adolescents | 1.0 | implant/30 months | 1 | implant/30 months | subdermal implant | Retisert | fluocinolone acetonide | PDR |
| Max dose for geriatric | 4.0 | application/day | 4 | application/day | skin/dermal | Retisert | fluocinolone acetonide | PDR |
| Max dose for geriatric | 3.0 | application/day | 3 | application/day | skin/dermal | Retisert | fluocinolone acetonide | PDR |
| Max dose for geriatric | 1.0 | ounce/day | 1 | ounce/day | skin/dermal | Retisert | fluocinolone acetonide | PDR |
| Max dose for geriatric | 30.0 | ml/day | 30 | ml/day | skin/dermal | Retisert | fluocinolone acetonide | PDR |
| Max dose for geriatric | 1.0 | implant/30 months | 1 | implant/30 months | subdermal implant | Retisert | fluocinolone acetonide | PDR |
| Max dose for geriatric | 1.0 | implant/30 months | 1 | implant/30 months | subdermal implant | Retisert | fluocinolone acetonide | PDR |