Basic Information
| Drug ID | DDPD00588 |

|
| Drug Name | Fluticasone propionate | |
| Molecular Weight | 500.571 | |
| Molecular Formula | C25H31F3O5S | |
| CAS Number | 80474-14-2 | |
| SMILES | [H][C@@]12C[C@@H](C)[C@](OC(=O)CC)(C(=O)SCF)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])C[C@H](F)C2=CC(=O)C=C[C@]12C | |
| External Links | ||
| DRUGBANK | DB00588 | |
| PubChem Compound | 444036 | |
| PDR | 1548 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
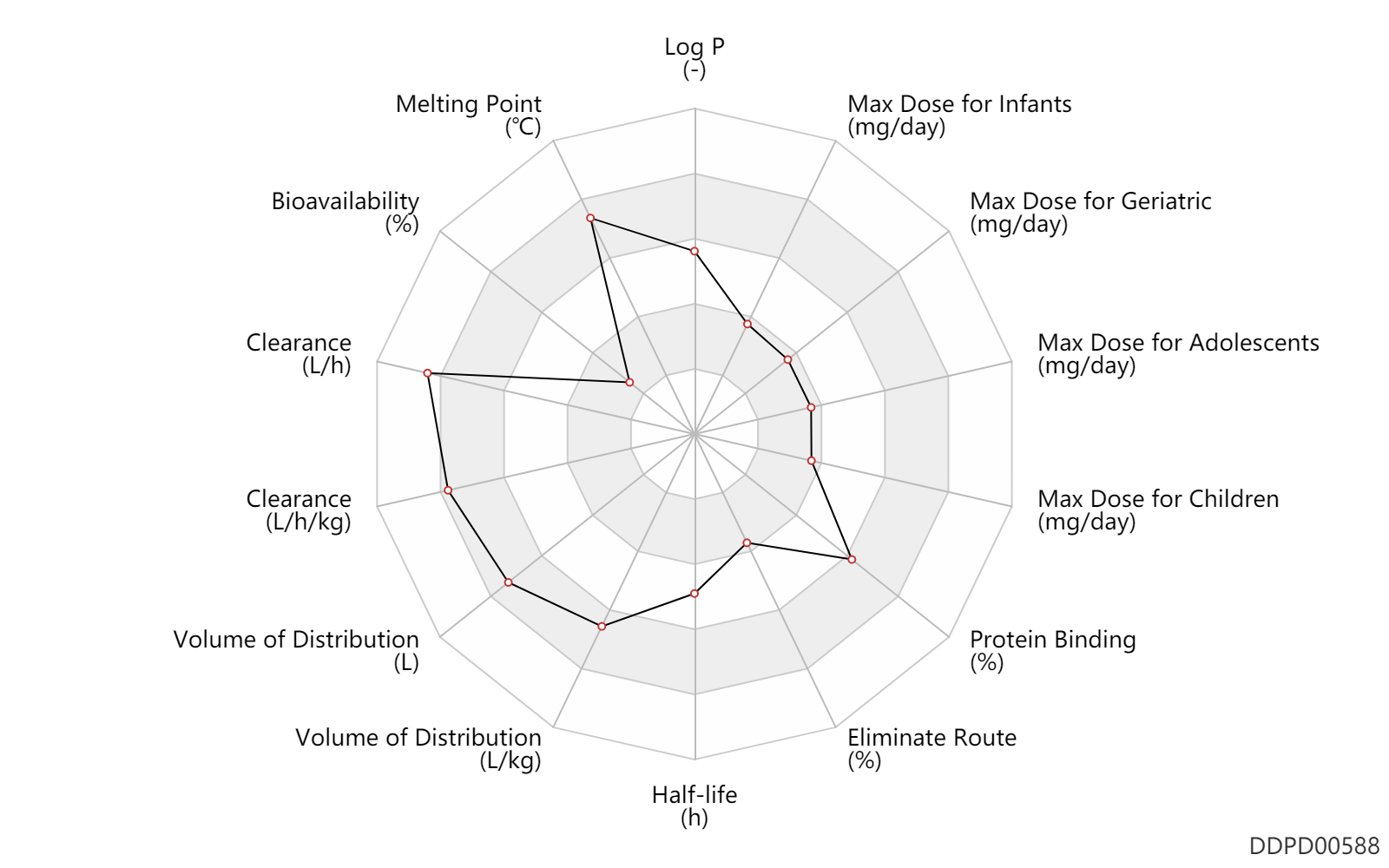
| Log P | 2.78 | - | 2.78 | - | [MSDS] |
| Melting Point | 267.0 | ℃ | 261-273 | ℃ | [MSDS] |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 2.0 | % | <2 | % | inhalation, IH; | DRUGBANK | Bioavailability | 1.0 | % | <1 | % | PO, oral; | DRUGBANK | Bioavailability | 9.0 | % | 9 | % | inhalation, IH; Caucasian; Male, men; | DRUGBANK |
| Clearance | 62.3 | L/h | 1093.0 | ml/min | DRUGBANK | Clearance | 63.9 | L/h | 63.9 | L/h | intravenous injection, IV; Caucasian; Male, men; | DRUGBANK | Clearance | 1.0 | L/h/kg | 17 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 4.2 | L/kg | 4.2 | L/kg | intravenous injection, IV; | DRUGBANK | Volume of Distribution | 557.0 | L | 557.0 | L | at steady state; intravenous injection, IV; normal,healthy; Caucasian; Male, men; | DRUGBANK | Volume of Distribution | 3.6 | L/kg | 3.6 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 7.8 | h | 7.8 | h | intravenous injection, IV; | DRUGBANK | Half-life | 14.0 | h | 14 | h | intravenous injection, IV; normal,healthy; Caucasian; Male, men; | DRUGBANK | Half-life | 10.8 | h | 10.8 | h | inhalation, IH; normal,healthy; Caucasian; Male, men; | DRUGBANK | Half-life | 6.0 | h | 6 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 5.0 | % | <5 | % | Urinary excretion; | DRUGBANK |
| Protein Binding | 99.0 | % | 99 | % | DRUGBANK | Protein Binding | 91.0 | % | 91 | % | nasal spray; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 2.0 | application/day | 2 | application/day | skin ointment | bid | Cutivate Lotion | fluticasone propionate | PDR |
| Max dose for neonates | 1.0 | application/day | 1 | application/day | skin/dermal | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 2.0 | appLication/day | 2 | appLication/day | skin ointment | bid | Cutivate Lotion | fluticasone propionate | PDR |
| Max dose for infants | 1.0 | appLication/day | 1 | appLication/day | skin/dermal | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.11 | mg/day | 110 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.2 | mg/day | 200 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 2.0 | mg/day | 2000 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.464 | mg/day | 464 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.2 | mg/day | 200 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 1.76 | mg/day | 1760 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 2.0 | appLication/day | 2 | appLication/day | skin ointment | bid | Cutivate Lotion | fluticasone propionate | PDR |
| Max dose for infants | 1.0 | appLication/day | 1 | appLication/day | skin/dermal | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.055 | mg/day | 55 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.2 | mg/day | 200 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.2 | mg/day | 200 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.4 | mg/day | 400 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.176 | mg/day | 176 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.352 | mg/day | 352 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.05 | mg/day | 50 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 2.0 | appLication/day | 2 | appLication/day | skin ointment | bid | Cutivate Lotion | fluticasone propionate | PDR |
| Max dose for infants | 1.0 | appLication/day | 1 | appLication/day | skin/dermal | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.055 | mg/day | 55 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.2 | mg/day | 200 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.2 | mg/day | 200 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 400.0 | 400 | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |||
| Max dose for infants | 0.176 | mg/day | 176 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.352 | mg/day | 352 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 2.0 | appLication/day | 2 | appLication/day | skin ointment | bid | Cutivate Lotion | fluticasone propionate | PDR |
| Max dose for infants | 1.0 | appLication/day | 1 | appLication/day | skin/dermal | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 0.055 | mg/day | 55 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for infants | 2.0 | appLication/day | 2 | appLication/day | skin ointment | bid | Cutivate Lotion | fluticasone propionate | PDR |
| Max dose for infants | 1.0 | appLication/day | 1 | appLication/day | skin/dermal | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for children | 2.0 | application/day | 2 | application/day | skin ointment | bid | Cutivate Lotion | fluticasone propionate | PDR |
| Max dose for children | 1.0 | application/day | 1 | application/day | skin/dermal | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for children | 0.11 | mg/day | 110 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for children | 0.2 | mg/day | 200 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for children | 2.0 | mg/day | 2000 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for children | 0.464 | mg/day | 464 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for children | 0.2 | mg/day | 200 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for children | 1.76 | mg/day | 1760 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for adolescents | 2.0 | application/day | 2 | application/day | skin ointment | bid | Cutivate Lotion | fluticasone propionate | PDR |
| Max dose for adolescents | 1.0 | application/day | 1 | application/day | skin/dermal | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for adolescents | 0.11 | mg/day | 110 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for adolescents | 0.2 | mg/day | 200 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for adolescents | 0.744 | mg/day | 744 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for adolescents | 2.0 | mg/day | 2000 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for adolescents | 0.464 | mg/day | 464 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for adolescents | 0.2 | mg/day | 200 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for adolescents | 1.76 | mg/day | 1760 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for geriatric | 2.0 | application/day | 2 | application/day | skin ointment | bid | Cutivate Lotion | fluticasone propionate | PDR |
| Max dose for geriatric | 1.0 | application/day | 1 | application/day | skin/dermal | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for geriatric | 0.11 | mg/day | 110 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for geriatric | 0.2 | mg/day | 200 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for geriatric | 0.744 | mg/day | 744 | mcg/day | nasal spray | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for geriatric | 2.0 | mg/day | 2000 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for geriatric | 0.464 | mg/day | 464 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for geriatric | 0.2 | mg/day | 200 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR | |
| Max dose for geriatric | 1.76 | mg/day | 1760 | mcg/day | inhalation, IH | Cutivate Lotion | fluticasone propionate | PDR |