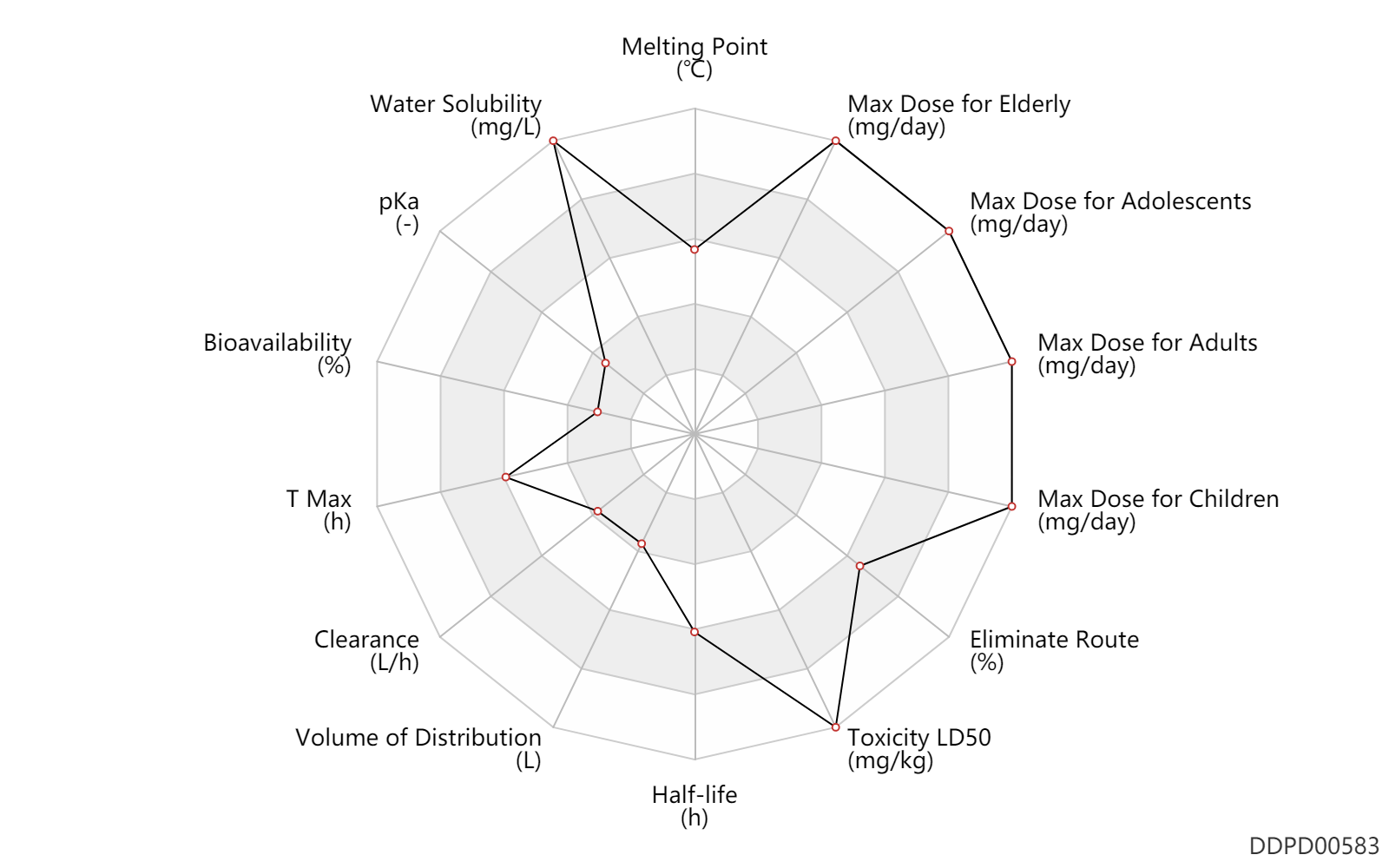
Basic Information
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Melting Point | 196.5 | ℃ | 195-198 | ℃ | Noguchi, J. and Sakota, N.; US. Patent 3,135,788; June 2,1964; assigned to Nihon Zoki Seiyaku Kabushikikaisha (Japan). |
| Water Solubility | 2500000.0 | mg/L | 2500 | mg/ml | DRUGBANK |
| pKa | 3.8 | - | 3.8 | - | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 15.0 | % | 15 | % | Tablet, PO, oral; Capsule, PO, Oral; | DRUGBANK |
| T Max | 3.3 | h | 3.3 | h | Tablet, PO, oral; Capsule, PO, Oral; | DRUGBANK |
| Clearance | 4.0 | L/h | 4.0 | L/h | Total clearance; | DRUGBANK |
| Volume of Distribution | 29.0 | L | 29.0±7.1 | L | Steady state volume of distribution; intravenous injection, IV; | DRUGBANK |
| Half-life | 17.4 | h | 17.4 | h | elimination half-life; | DRUGBANK |
| Toxicity LD50 | 8000.0 | mg/kg | 8.0 | g/kg | PO, oral; mouse; | T3DB |
| Eliminate Route | 73.1 | % | 73.1±16 | % | Urinary excretion; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 3000.0 | mg/day | 3 | g/day | PO, oral | Carnitor Oral Solution, Tablets, and SF Sugar-Free Oral Solution | levocarnitine | PDR |
| Max dose for adolescents | 3000.0 | mg/day | 3 | g/day | PO, oral | Carnitor Oral Solution, Tablets, and SF Sugar-Free Oral Solution | levocarnitine | PDR |
| Max dose for adults | 6000.0 | mg/day | 6 | g/day | PO, oral | Carnitor Oral Solution, Tablets, and SF Sugar-Free Oral Solution | levocarnitine | PDR |
| Max dose for elderly | 6000.0 | mg/day | 6 | g/day | PO, oral | Carnitor Oral Solution, Tablets, and SF Sugar-Free Oral Solution | levocarnitine | PDR |