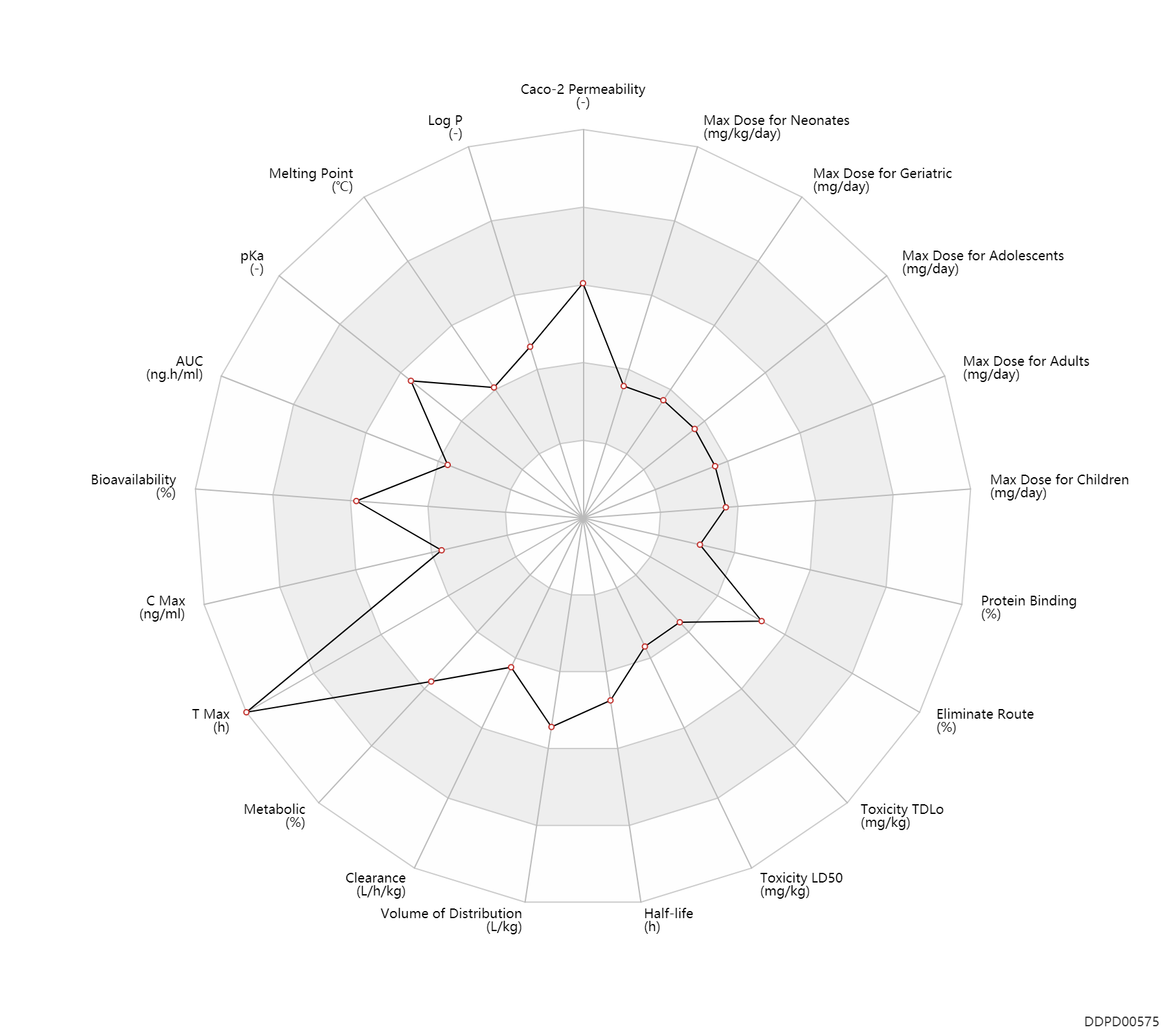
Basic Information
| Drug ID | DDPD00575 |

|
| Drug Name | Clonidine | |
| Molecular Weight | 230.094 | |
| Molecular Formula | C9H9Cl2N3 | |
| CAS Number | 4205-90-7 | |
| SMILES | ClC1=CC=CC(Cl)=C1NC1=NCCN1 | |
| External Links | ||
| DRUGBANK | DB00575 | |
| T3DB | T3D2829 | |
| PubChem Compound | 2803 | |
| PDR | 1744 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Caco-2 Permeability | -4.59 | - | -4.59 | - | ADME Research, USCD |
| Log P | 1.59 | - | 1.59 | - | HANSCH,C & LEO,AJ (1985) |
| Melting Point | 130.0 | ℃ | 130 | ℃ | http://www.chemspider.com/Chemical-Structure.2701.html?rid=816613e6-a54a-46f0-8152-4a93e09e4170 |
| pKa | 8.05 | - | 8.05 | - | KONTTURI,K & MURTOMAKI,L (1992) |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| AUC | 5.6 | ng.h/ml | 5606.78 | pg.h/ml | Tablet, PO, oral; | DRUGBANK | |
| Bioavailability | 71.0 | % | 55-87 | % | Tablet, PO, oral; | DRUGBANK | Bioavailability | 95.0 | % | 95.0 | % | PO, oral; | The Pharmacological Basis of Therapeutics | Bioavailability | 60.0 | % | 60.0 | % | Transdermal preparations; | The Pharmacological Basis of Therapeutics |
| C Max | 0.40 | ng/ml | 400.72 | pg/ml | Tablet, PO, oral; | DRUGBANK | C Max | 0.80 | ng/ml | 0.8 | ng/ml | PO, oral; | The Pharmacological Basis of Therapeutics | C Max | 0.35 | ng/ml | 0.3-0.4 | ng/ml | Transdermal preparations; | The Pharmacological Basis of Therapeutics |
| T Max | 1.3 | h | 1-1.5 | h | PO, oral; food; | food → ; | DRUGBANK | T Max | 2.0 | h | 2.0 | h | PO, oral; | The Pharmacological Basis of Therapeutics | T Max | 72.0 | h | 72.0 | h | Transdermal preparations; | The Pharmacological Basis of Therapeutics |
| Metabolic | 50.0 | % | <50 | % | Liver metabolism; Inactive metabolite; | DRUGBANK | |
| Clearance | 0.19 | L/h/kg | 1.9-4.3 | ml/min/kg | DRUGBANK | Clearance | 0.19 | L/h/kg | 3.1±1.2 | ml/min/kg | RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics | Clearance | 0.24 | L/h/kg | 4 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 2.1 | L/kg | 2.1±0.4 | L/kg | DRUGBANK | Volume of Distribution | 2.1 | L/kg | 2.1±0.4 | L/kg | The Pharmacological Basis of Therapeutics | Volume of Distribution | 3.3 | L/kg | 3.3 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 0.50 | h | 30 | min | elimination half-life; IM,intramuscular injection; | DRUGBANK | Half-life | 14.5 | h | 6-23 | h | DRUGBANK | Half-life | 12.0 | h | 12±7 | h | RD, renal impairment, Renal disease,including uremia ↑ ; | The Pharmacological Basis of Therapeutics | Half-life | 7.6 | h | 7.6 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 125.0 | mg/kg | 125.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 150.0 | mg/kg | 150.0 | mg/kg | PO, oral; Rattus, Rat; | T3DB | Toxicity LD50 | 30.0 | mg/kg | 30.0 | mg/kg | PO, oral; dog; | T3DB |
| Toxicity TDLo | 0.0700 | mg/kg | 70.0 | ug/kg | Children; | DRUGBANK | Toxicity TDLo | 0.13 | mg/kg | 126.0 | ug/kg | Female, women; | DRUGBANK | Toxicity TDLo | 0.0690 | mg/kg | 69.0 | ug/kg | Male, men; | DRUGBANK |
| Eliminate Route | 20.0 | % | 20 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 50.0 | % | ~50 | % | Urinary excretion; Unchanged drug; | DRUGBANK | Eliminate Route | 62.0 | % | 62±11 | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 30.0 | % | 20-40 | % | DRUGBANK | Protein Binding | 20.0 | % | 20 | % | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 0.012 | mg/kg/day | 12 | mcg/kg/day | PO, oral | Catapres | clonidine hydrochloride | PDR |
| Max dose for neonates | 0.012 | mg/kg/day | 12 | mcg/kg/day | PO, oral | Catapres | clonidine hydrochloride | PDR |
| Max dose for infants | 0.004 | mg/kg | 4 | mcg/kg | PO, oral | Catapres | clonidine hydrochloride | PDR |
| Max dose for children | 0.4 | mg/day | 0.4 | mg/day | Tablet,PO,oral | Catapres | clonidine hydrochloride | PDR |
| Max dose for children | 2.4 | mg/day | 2.4 | mg/day | Tablet,PO,oral | Catapres | clonidine hydrochloride | PDR |
| Max dose for children | 0.4 | mg/day | 0.4 | mg/day | Tablet,PO,oral | Catapres | clonidine hydrochloride | PDR |
| Max dose for children | 0.9 | mg/day | 0.9 | mg/day | Tablet,PO,oral | Catapres | clonidine hydrochloride | PDR |
| Max dose for children | 0.9 | mg/day | 0.9 | mg/day | Tablet,PO,oral | Catapres | clonidine hydrochloride | PDR |
| Max dose for adolescents | 0.4 | mg/day | 0.4 | mg/day | Tablet,PO,oral | Catapres | clonidine hydrochloride | PDR |
| Max dose for adolescents | 2.4 | mg/day | 2.4 | mg/day | Tablet,PO,oral | Catapres | clonidine hydrochloride | PDR |
| Max dose for adults | 2.4 | mg/day | 2.4 | mg/day | Tablet,PO,oral | Catapres | clonidine hydrochloride | PDR |
| Max dose for adults | 2.0 | patches/week | 2 | patches/week | Transdermal preparations | Catapres | clonidine hydrochloride | PDR |
| Max dose for adults | 0.6 | mg/day | 0.6 | mg/day | Transdermal preparations | Catapres | clonidine hydrochloride | PDR |
| Max dose for adults | 0.96 | mg/day | 40 | mcg/h | epidural administration | Catapres | clonidine hydrochloride | PDR |
| Max dose for geriatric | 2.4 | mg/day | 2.4 | mg/day | Tablet,PO,oral | Catapres | clonidine hydrochloride | PDR |
| Max dose for geriatric | 2.0 | patches/week | 2 | patches/week | Transdermal preparations | Catapres | clonidine hydrochloride | PDR |
| Max dose for geriatric | 0.6 | mg/day | 0.6 | mg/day | Transdermal preparations | Catapres | clonidine hydrochloride | PDR |
| Max dose for geriatric | 0.96 | mg/day | 40 | mcg/hour | epidural administration | Catapres | clonidine hydrochloride | PDR |