Basic Information
| Drug ID | DDPD00573 |

|
| Drug Name | Fenoprofen | |
| Molecular Weight | 242.2699 | |
| Molecular Formula | C15H14O3 | |
| CAS Number | 29679-58-1 | |
| SMILES | CC(C(O)=O)C1=CC(OC2=CC=CC=C2)=CC=C1 | |
| External Links | ||
| DRUGBANK | DB00573 | |
| PubChem Compound | 3342 | |
| PDR | 1509 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
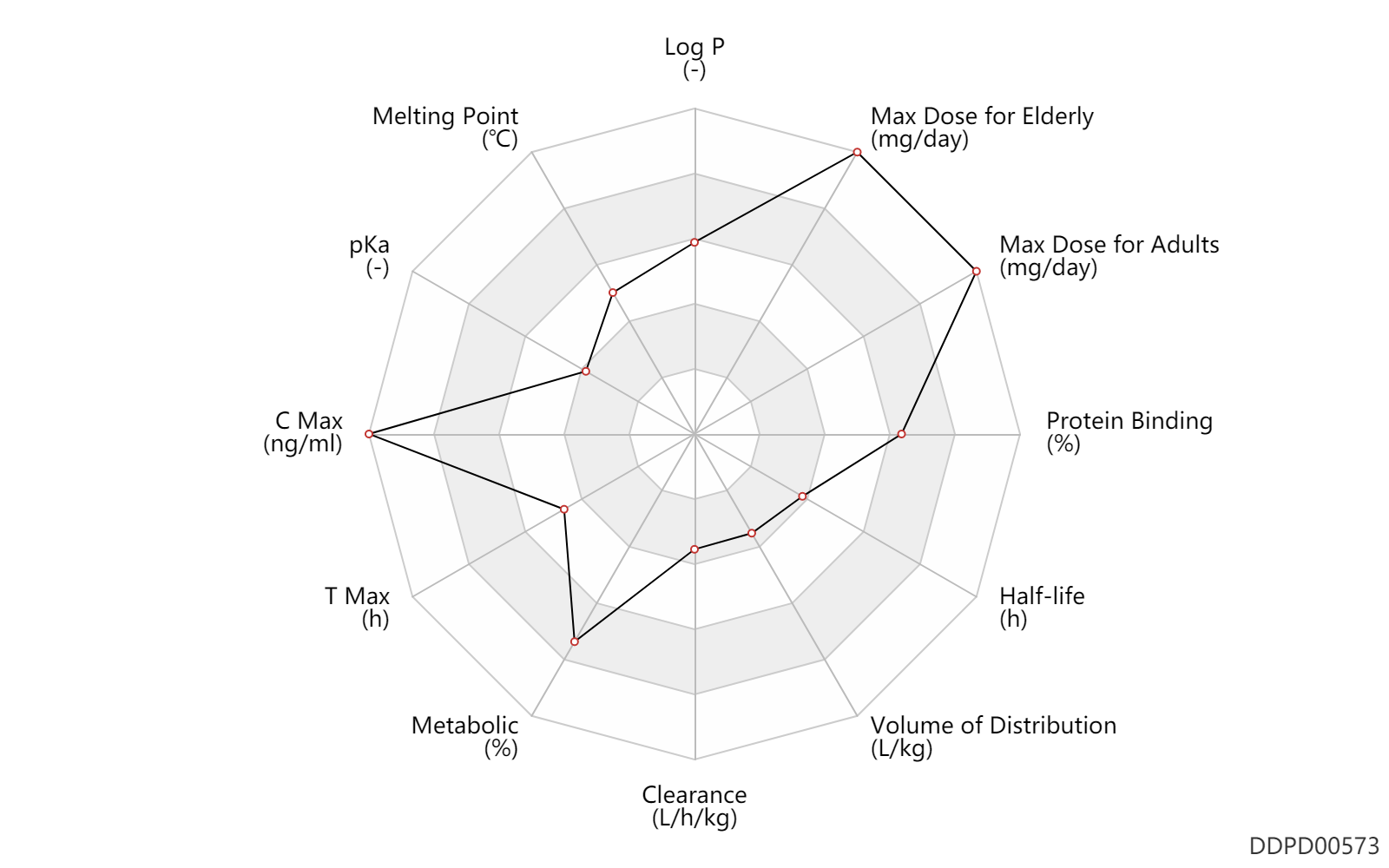
| Log P | 3.1 | - | 3.1 | - | DRUGBANK |
| Melting Point | 169.5 | ℃ | 168-171 | ℃ | Marshall, W.S.; U.S. Patent 3,600,437; August 17, 1971; assigned to Eli Lilly and Company. |
| pKa | 4.5 | - | 4.5 | - | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| C Max | 50000.0 | ng/ml | 50.0 | mcg/ml | Oral single dose; | DRUGBANK |
| T Max | 2.0 | h | 2 | h | Oral single dose; | DRUGBANK |
| Metabolic | 90.0 | % | 90 | % | PO, oral; | DRUGBANK |
| Clearance | 0.0360 | L/h/kg | 0.6 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 0.10 | L/kg | 0.1 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 3.0 | h | ~3 | h | elimination half-life; | DRUGBANK | Half-life | 2.2 | h | 2.24 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Protein Binding | 99.0 | % | 99 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 3200.0 | mg/day | 3200 | mg/day | PO, oral | Nalfon | fenoprofen calcium | PDR |
| Max dose for elderly | 3200.0 | mg/day | 3200 | mg/day | PO, oral | Nalfon | fenoprofen calcium | PDR |