Basic Information
| Drug ID | DDPD00551 |

|
| Drug Name | Acetohydroxamic acid | |
| Molecular Weight | 75.0666 | |
| Molecular Formula | C2H5NO2 | |
| CAS Number | 546-88-3 | |
| SMILES | CC(=O)NO | |
| External Links | ||
| DRUGBANK | DB00551 | |
| PubChem Compound | 1990 | |
| PDR | 3224 | |
| Drugs.com | Drugs.com Drug Page | |
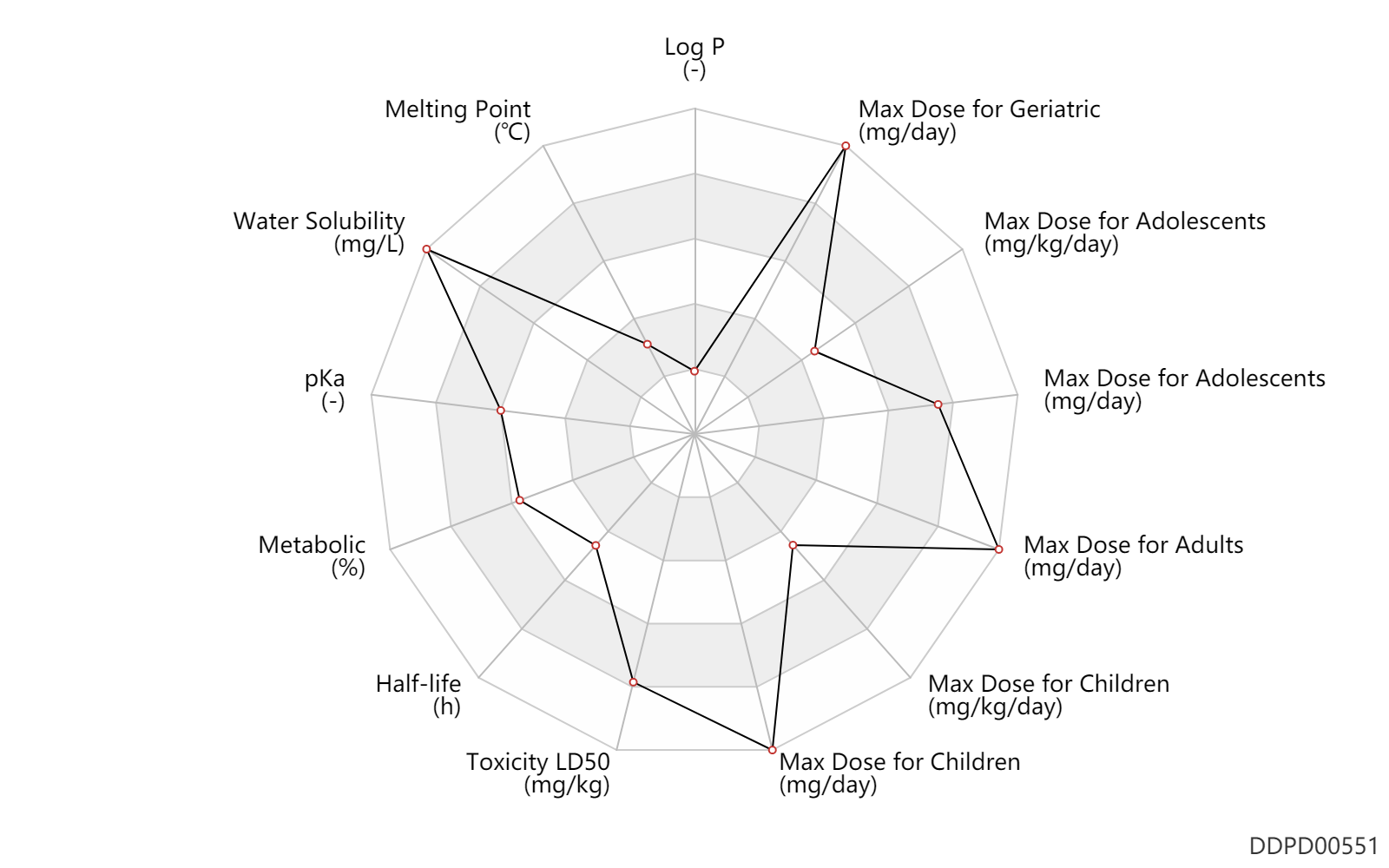
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Log P | -1.59 | - | -1.59 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 90.5 | ℃ | 90.5 | ℃ | PhysProp |
| Water Solubility | 1000000.0 | mg/L | 1000000 | mg/L | DRUGBANK |
| pKa | 8.7 | - | 8.7 | - | SERJEANT,EP & DEMPSEY,B (1979) |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Metabolic | 50.0 | % | 35-65 | % | Urinary excretion; Unchanged drug; | DRUGBANK |
| Half-life | 7.5 | h | 5-10 | h | patients; normal renal function; | DRUGBANK |
| Toxicity LD50 | 4800.0 | mg/kg | 4.8 | g/kg | PO, oral; Rattus, Rat; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 15.0 | mg/kg/day | 15 | mg/kg/day | PO, oral | Lithostat | acetohydroxamic acid | PDR |
| Max dose for children | 1500.0 | mg/day | 1.5 | g/day | PO, oral | Lithostat | acetohydroxamic acid | PDR |
| Max dose for adolescents | 15.0 | mg/kg/day | 15 | mg/kg/day | PO, oral | Lithostat | acetohydroxamic acid | PDR |
| Max dose for adolescents | 1500.0 | mg/day | 1.5 | g/day | PO, oral | Lithostat | acetohydroxamic acid | PDR |
| Max dose for adults | 1500.0 | mg/day | 1.5 | g/day | PO, oral | Lithostat | acetohydroxamic acid | PDR |
| Max dose for geriatric | 1500.0 | mg/day | 1.5 | g/day | PO, oral | Lithostat | acetohydroxamic acid | PDR |