Basic Information
| Drug ID | DDPD00549 |

|
| Drug Name | Zafirlukast | |
| Molecular Weight | 575.675 | |
| Molecular Formula | C31H33N3O6S | |
| CAS Number | 107753-78-6 | |
| SMILES | COC1=CC(=CC=C1CC1=CN(C)C2=C1C=C(NC(=O)OC1CCCC1)C=C2)C(=O)NS(=O)(=O)C1=CC=CC=C1C | |
| External Links | ||
| DRUGBANK | DB00549 | |
| PubChem Compound | 5717 | |
| PDR | 2054 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
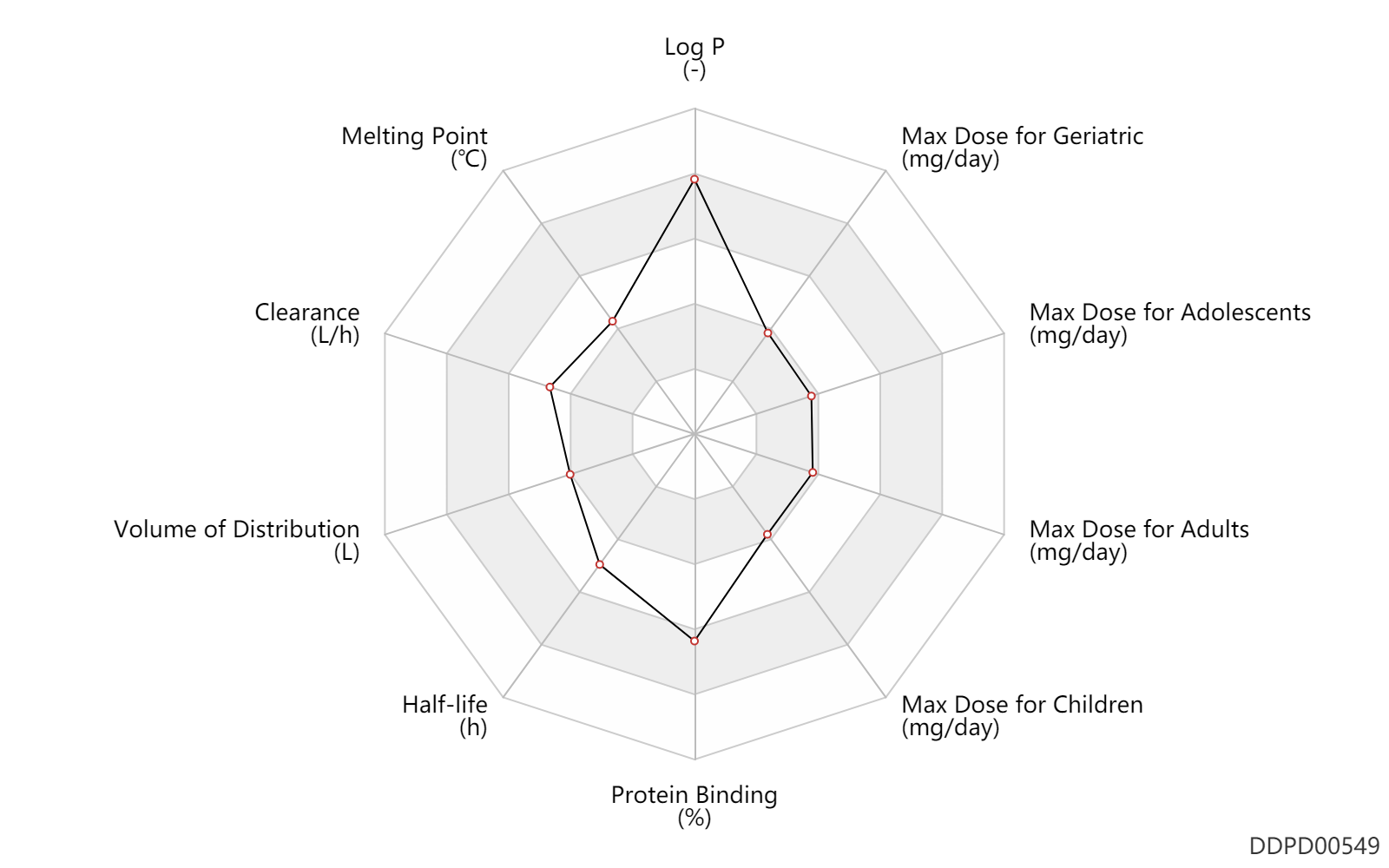
| Log P | 5.4 | - | 5.4 | - | DRUGBANK |
| Melting Point | 139.0 | ℃ | 139 | ℃ | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Clearance | 20.0 | L/h | 20.0 | L/h | Total clearance; PO, oral; | DRUGBANK | Clearance | 10.3 | L/h | 9.2-11.4 | L/h | Children; | DRUGBANK |
| Volume of Distribution | 70.0 | L | 70.0 | L | DRUGBANK | |
| Half-life | 10.0 | h | 10 | h | DRUGBANK | |
| Protein Binding | 99.0 | % | 99 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 40.0 | mg/day | 40 | mg/day | PO, oral | Accolate | zafirlukast | PDR |
| Max dose for children | 20.0 | mg/day | 20 | mg/day | PO, oral | Accolate | zafirlukast | PDR |
| Max dose for adolescents | 40.0 | mg/day | 40 | mg/day | PO, oral | Accolate | zafirlukast | PDR |
| Max dose for adults | 40.0 | mg/day | 40 | mg/day | PO, oral | Accolate | zafirlukast | PDR |
| Max dose for geriatric | 40.0 | mg/day | 40 | mg/day | PO, oral | Accolate | zafirlukast | PDR |