Basic Information
| Drug ID | DDPD00543 |

|
| Drug Name | Amoxapine | |
| Molecular Weight | 313.781 | |
| Molecular Formula | C17H16ClN3O | |
| CAS Number | 14028-44-5 | |
| SMILES | ClC1=CC2=C(OC3=CC=CC=C3N=C2N2CCNCC2)C=C1 | |
| External Links | ||
| DRUGBANK | DB00543 | |
| T3DB | T3D2819 | |
| PubChem Compound | 2170 | |
| PDR | 2079 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
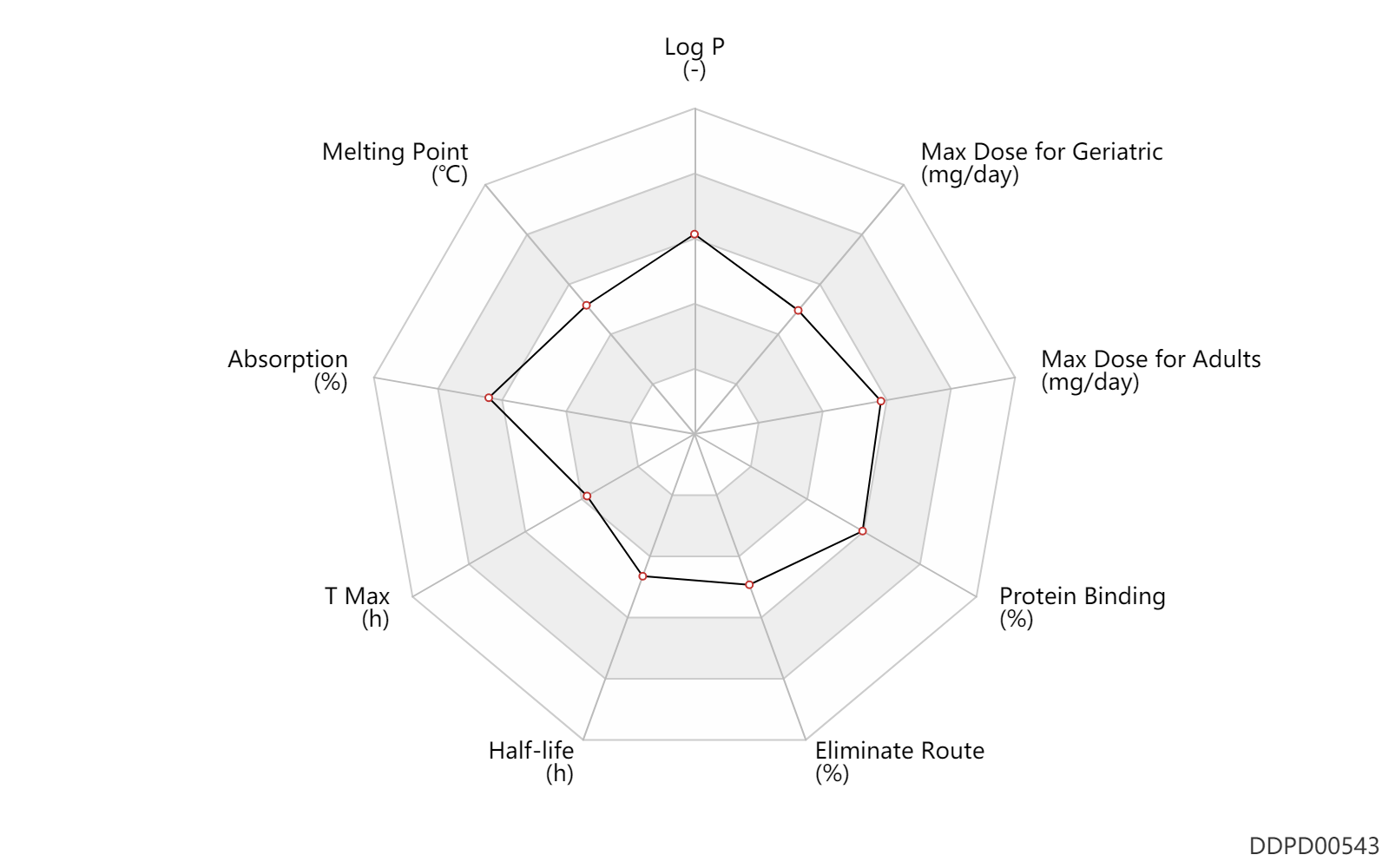
| Log P | 3.4 | - | 3.4 | - | DRUGBANK |
| Melting Point | 175.5 | ℃ | 175-176 | ℃ | Howell, C.F., Hardy, R.A., Jr. and Quinones, N.Q.; US. Patent 3,663,696; May 16, 1972; assigned to American Cyanamid Company Howell, C.F., Hardy, R.A., Jr. and Quinones, N.Q.; U.S. Patent 3,681,357; August 1, 1972; assigned to American Cyanamid Company |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Absorption | 100.0 | % | ~100 | % | Oral single dose; | DRUGBANK |
| T Max | 1.2 | h | 1-2 | h | Oral single dose; | DRUGBANK |
| Half-life | 8.0 | h | 8 | h | DRUGBANK | |
| Eliminate Route | 64.5 | % | 60-69 | % | Urinary excretion; Oral single dose; | DRUGBANK | Eliminate Route | 5.0 | % | <5 | % | Urinary excretion; Oral single dose; Unchanged drug; | DRUGBANK |
| Protein Binding | 90.0 | % | ~90 | % | plasma proteins; high protein binding; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 400.0 | mg/day | 400 | mg/day | PO, oral | Amoxapine | amoxapine | PDR |
| Max dose for adults | 600.0 | mg/day | 600 | mg/day | PO, oral | Amoxapine | amoxapine | PDR |
| Max dose for geriatric | 300.0 | mg/day | 300 | mg/day | PO, oral | Amoxapine | amoxapine | PDR |