Basic Information
| Drug ID | DDPD00518 |

|
| Drug Name | Albendazole | |
| Molecular Weight | 265.331 | |
| Molecular Formula | C12H15N3O2S | |
| CAS Number | 54965-21-8 | |
| SMILES | CCCSC1=CC2=C(C=C1)N=C(NC(=O)OC)N2 | |
| External Links | ||
| DRUGBANK | DB00518 | |
| T3DB | T3D3476 | |
| PubChem Compound | 2082 | |
| PDR | 2202 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
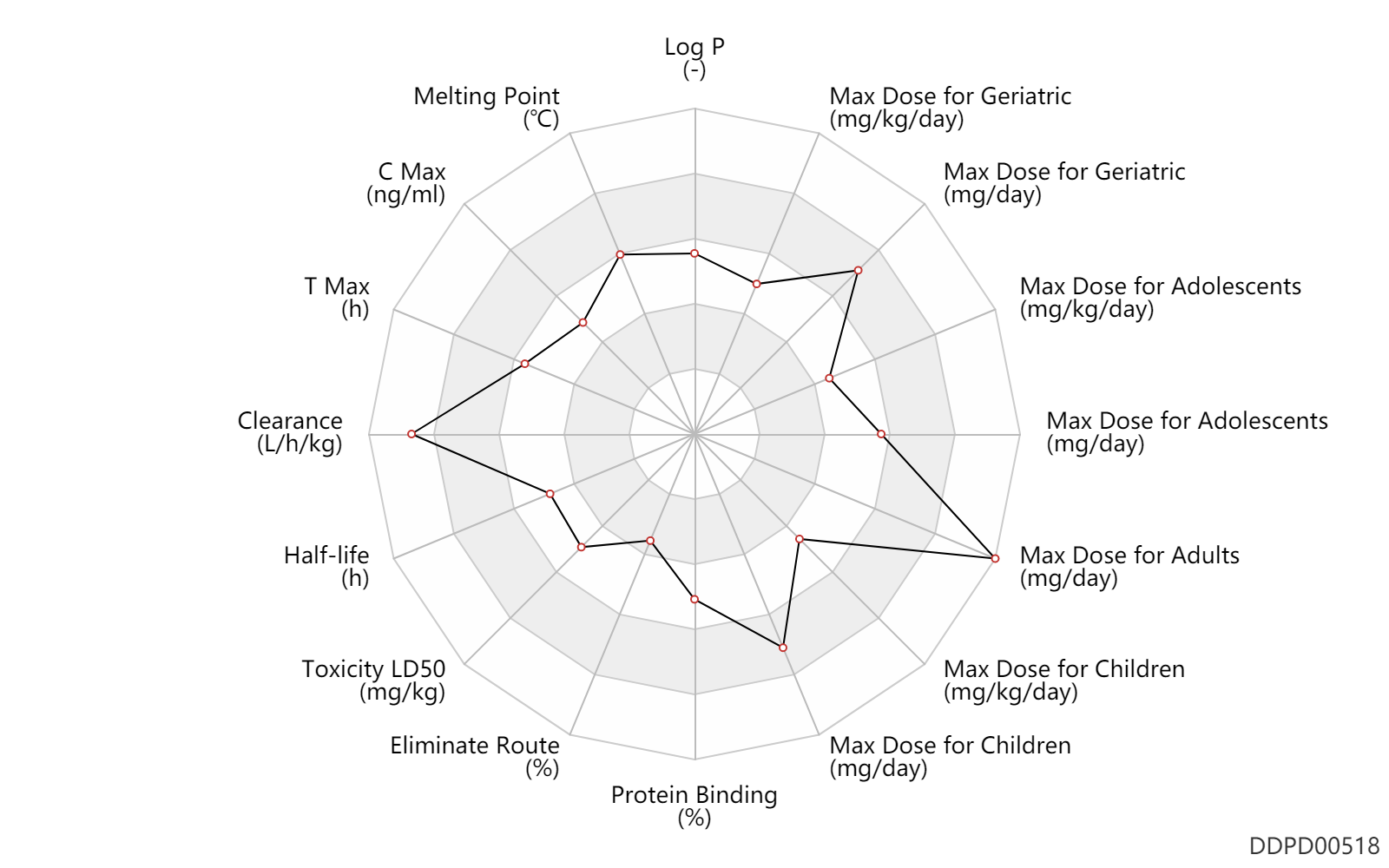
| Log P | 2.7 | - | 2.7 | - | DRUGBANK |
| Melting Point | 209.0 | ℃ | 208-210 | ℃ | Gyurik, R.J. and Theodorides, VJ.; US. Patent 3,915,986; October 28,1975; assigned to Smith Kline Corp. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| C Max | 1150.0 | ng/ml | 0.50-1.8 | mcg/ml | PO, oral; | The Pharmacological Basis of Therapeutics |
| T Max | 3.0 | h | 2-4 | h | PO, oral; | The Pharmacological Basis of Therapeutics |
| Clearance | 1.2 | L/h/kg | 10.5-30.7 | ml/min/kg | apparent clearance; Male, men; Female, women; adults; | The Pharmacological Basis of Therapeutics |
| Half-life | 10.0 | h | 8-12 | h | elimination half-life; Single dose; | DRUGBANK | Half-life | 8.0 | h | 8(6-15) | h | The Pharmacological Basis of Therapeutics |
| Toxicity LD50 | 1500.0 | mg/kg | 1500.0 | mg/kg | PO, oral; mouse; | T3DB |
| Eliminate Route | 1.0 | % | <1 | % | Urinary excretion; PO, oral; adults; human, homo sapiens; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 70.0 | % | 70 | % | plasma proteins; | DRUGBANK | Protein Binding | 70.0 | % | 70 | % | PO, oral; adults; human, homo sapiens; | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 15.0 | mg/kg/day | 15 | mg/kg/day | PO, oral | Albenza | albendazole | PDR |
| Max dose for children | 800.0 | mg/day | 800 | mg/day | PO, oral | Albenza | albendazole | PDR |
| Max dose for adolescents | 15.0 | mg/kg/day | 15 | mg/kg/day | PO, oral | Albenza | albendazole | PDR |
| Max dose for adolescents | 800.0 | mg/day | 800 | mg/day | PO, oral | Albenza | albendazole | PDR |
| Max dose for adults | 15.0 | mg/kg/day | 15 | mg/kg/day | PO, oral | Albenza | albendazole | PDR |
| Max dose for adults | 800.0 | mg/day | 800 | mg/day | PO, oral | Albenza | albendazole | PDR |
| Max dose for adults | 3200.0 | mg/day | 3200 | mg/day | PO, oral | Albenza | albendazole | PDR |
| Max dose for geriatric | 15.0 | mg/kg/day | 15 | mg/kg/day | PO, oral | Albenza | albendazole | PDR |
| Max dose for geriatric | 800.0 | mg/day | 800 | mg/day | PO, oral | Albenza | albendazole | PDR |