Basic Information
| Drug ID | DDPD00489 |

|
| Drug Name | Sotalol | |
| Molecular Weight | 272.364 | |
| Molecular Formula | C12H20N2O3S | |
| CAS Number | 3930-20-9 | |
| SMILES | CC(C)NCC(O)C1=CC=C(NS(C)(=O)=O)C=C1 | |
| External Links | ||
| DRUGBANK | DB00489 | |
| PubChem Compound | 5253 | |
| PDR | 23936 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
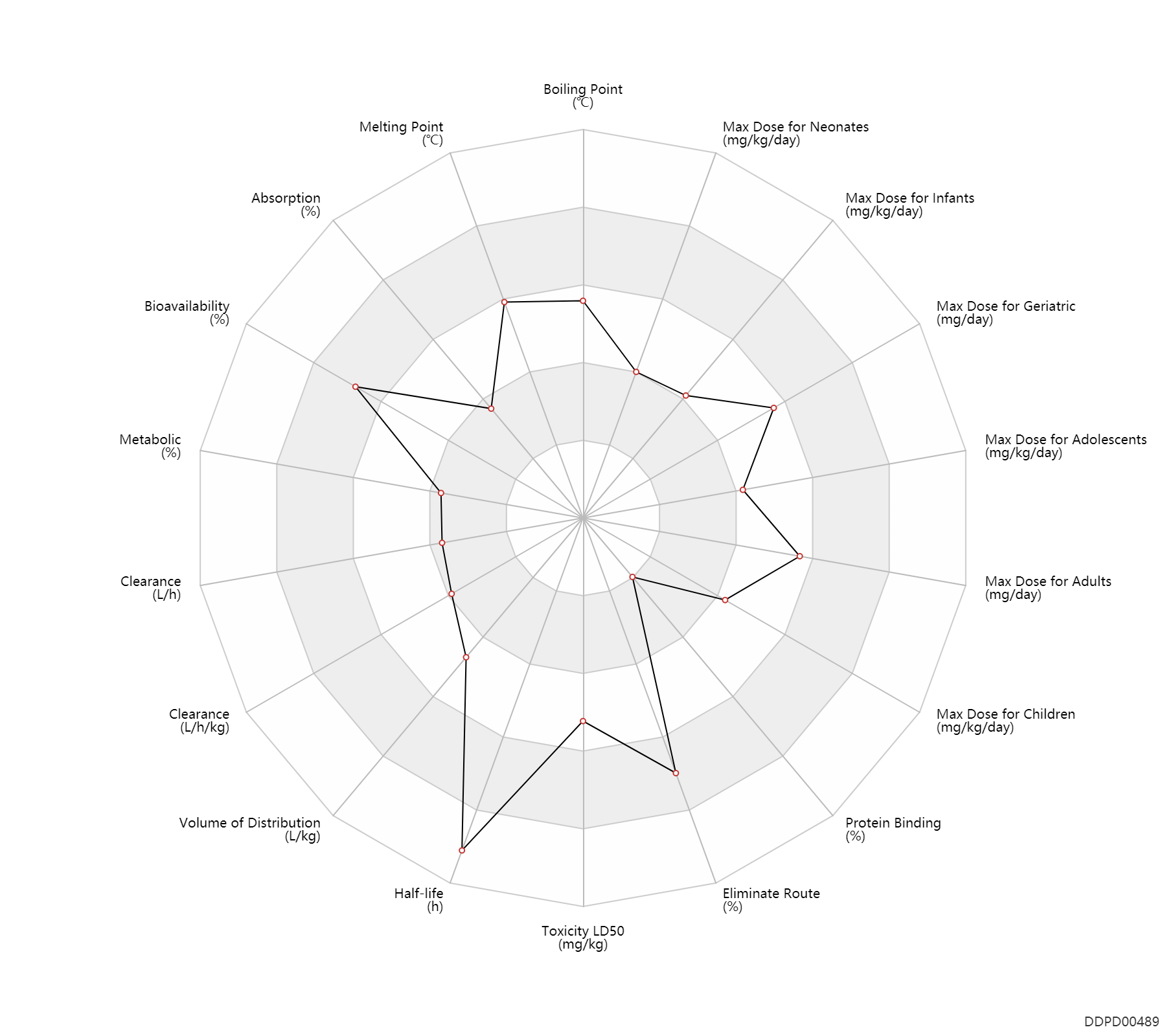
| Boiling Point | 443.3 | ℃ | 443.3 | ℃ | http://www.chemspider.com/Chemical-Structure.5063.html |
| Melting Point | 206.75 | ℃ | 206.75 | ℃ | http://www.chemspider.com/Chemical-Structure.5063.html |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Absorption | 18.0 | % | <18 | % | PO, oral; food; | DRUGBANK |
| Bioavailability | 95.0 | % | 90-100 | % | DRUGBANK | |
| C Max | 6.3 | null | 6.25±2.19 | null | PO, oral; food; | DRUGBANK |
| Metabolic | 0 | % | 0 | % | DRUGBANK | |
| Clearance | 6.8 | L/h | 6.78±2.72 | L/h | Plasma clearance; normal,healthy; patients; | DRUGBANK | Clearance | 5.0 | L/h | 4.99±1.43 | L/h | Renal clearance; normal,healthy; patients; | DRUGBANK | Clearance | 2.7 | L/h | 2.74±0.53 | L/h | Plasma clearance; moderate renal function; patients; | DRUGBANK | Clearance | 2.0 | L/h | 2.00±0.67 | L/h | Renal clearance; moderate renal function; patients; | DRUGBANK | Clearance | 1.6 | L/h | 1.56±0.44 | L/h | Plasma clearance; severe renal function; patients; | DRUGBANK | Clearance | 0.65 | L/h | 0.65±0.31 | L/h | Renal clearance; severe renal function; patients; | DRUGBANK | Clearance | 0.65 | L/h | 0.65±0.20 | L/h | Plasma clearance; renal insufficiency; patients; | DRUGBANK | Clearance | 0.27 | L/h | 0.27±0.13 | L/h | Renal clearance; renal insufficiency; patients; | DRUGBANK | Clearance | 0.12 | L/h/kg | 2 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 1.8 | L/kg | 1.2-2.4 | L/kg | Apparent volume of distribution; | DRUGBANK | Volume of Distribution | 1.3 | L/kg | 1.3 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 15.0 | h | 10-20 | h | elimination half-life; normal,healthy; patients; | DRUGBANK | Half-life | 17.5 | h | 17.5±0.97 | h | normal renal function; patients; | DRUGBANK | Half-life | 22.7 | h | 22.7±6.4 | h | mild renal function; moderate renal function; patients; | DRUGBANK | Half-life | 64.0 | h | 64±27.2 | h | severe renal function; patients; | DRUGBANK | Half-life | 97.9 | h | 97.9±57.3 | h | renal insufficiency; | DRUGBANK | Half-life | 6.3 | h | 6.3 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 3450.0 | mg/kg | 3450.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 680.0 | mg/kg | 680.0 | mg/kg | Intraperitoneal, IP; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 2600.0 | mg/kg | 2600.0 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 670.0 | mg/kg | 670.0 | mg/kg | Intraperitoneal, IP; mouse; | DRUGBANK |
| Eliminate Route | 85.0 | % | 80-90 | % | Urinary excretion; Unchanged drug; | DRUGBANK |
| Protein Binding | 0 | % | 0 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 10.0 | mg/kg/day | 10 | mg/kg/day | PO, oral | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for infants | 10.0 | mg/kg/day | 10 | mg/kg/day | PO, oral | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for children | 180.0 | mg/m2/day | 180 | mg/m2/day | PO, oral | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for children | 10.0 | mg/kg/day | 10 | mg/kg/day | PO, oral | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for children | 10.0 | mg/kg/day | 10 | mg/kg/day | PO, oral | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for adolescents | 180.0 | mg/m2/day | 180 | mg/m2/day | PO, oral | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for adolescents | 10.0 | mg/kg/day | 10 | mg/kg/day | PO, oral | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for adults | 320.0 | mg/day | 320 | mg/day | PO, oral | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for adults | 300.0 | mg/day | 300 | mg/day | intravenous injection, IV | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for adults | 640.0 | mg/day | 640 | mg/day | PO, oral | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for adults | 600.0 | mg/day | 600 | mg/day | intravenous injection, IV | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for geriatric | 320.0 | mg/day | 320 | mg/day | PO, oral | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for geriatric | 300.0 | mg/day | 300 | mg/day | intravenous injection, IV | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for geriatric | 640.0 | mg/day | 640 | mg/day | PO, oral | Betapace/Betapace AF | sotalol hydrochloride | PDR |
| Max dose for geriatric | 600.0 | mg/day | 600 | mg/day | intravenous injection, IV | Betapace/Betapace AF | sotalol hydrochloride | PDR |