Basic Information
| Drug ID | DDPD00477 |

|
| Drug Name | Chlorpromazine | |
| Molecular Weight | 318.864 | |
| Molecular Formula | C17H19ClN2S | |
| CAS Number | 50-53-3 | |
| SMILES | CN(C)CCCN1C2=CC=CC=C2SC2=C1C=C(Cl)C=C2 | |
| External Links | ||
| DRUGBANK | DB00477 | |
| T3DB | T3D2806 | |
| PubChem Compound | 2726 | |
| PDR | 1696 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
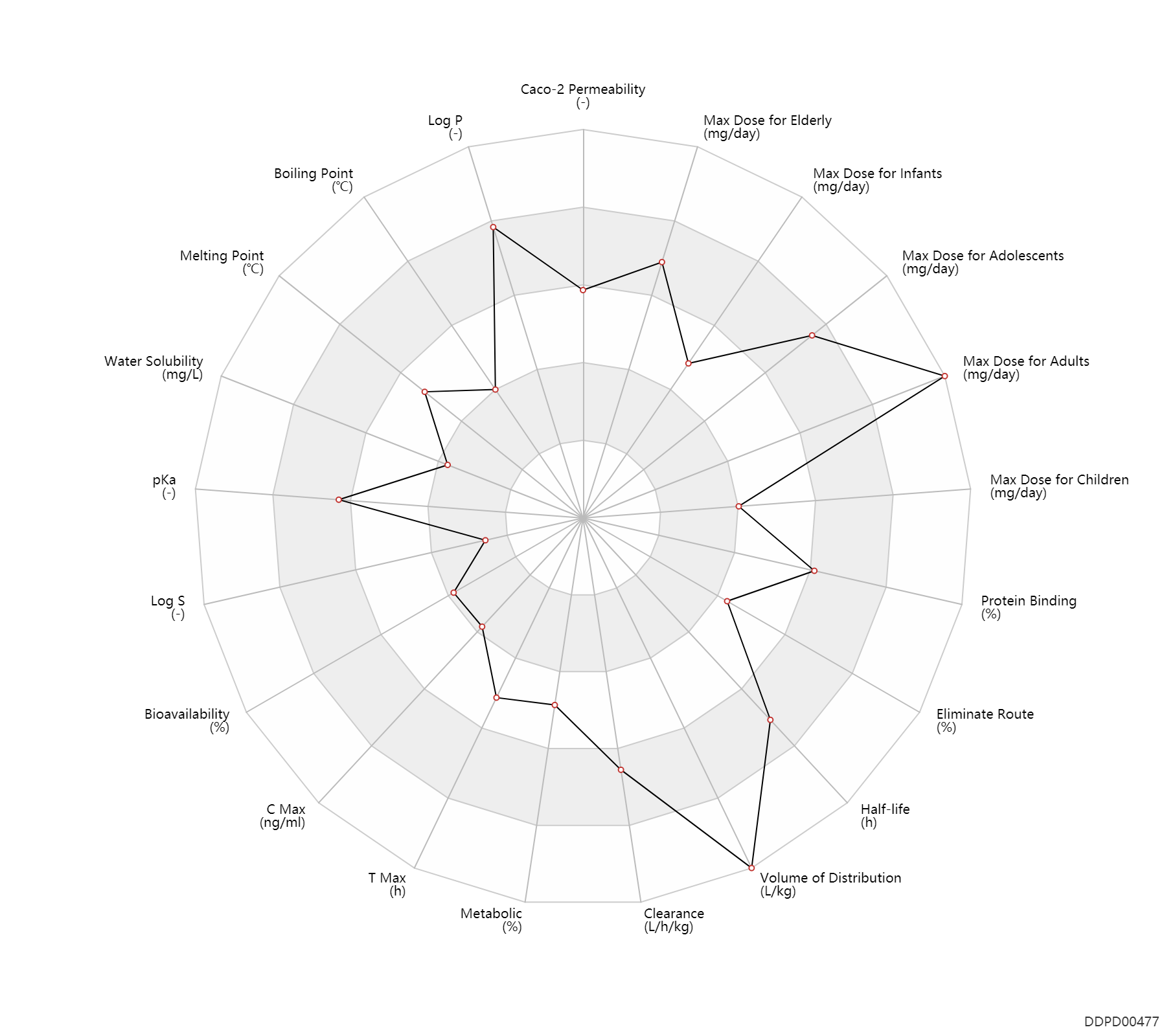
| Caco-2 Permeability | -4.7 | - | -4.7 | - | ADME Research, USCD |
| Log P | 5.41 | - | 5.41 | - | AVDEEF,A (1995) |
| Boiling Point | 202.5 | ℃ | 200-205 | ℃ | PhysProp |
| Melting Point | 177.5 | ℃ | 177-178 | ℃ | Charpentier, P.; U S . Patent 2,645,640; July 14, 1953; assigned to Societe des Usines Chimiques Rhone-Poulenc, France |
| Water Solubility | 2.55 | mg/L | 2.55 | mg/L | YALKOWSKY,SH & DANNENFELSER,RM 1992) |
| pKa | 9.3 | - | 9.3 | - | HANSCH,C & LEO,AJ (1985) |
| Log S | -5.01 | - | -5.01 | - | ADME Research, USCD |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 32.0 | % | 32±19 | % | PO, oral; | The Pharmacological Basis of Therapeutics | |
| C Max | 87.5 | ng/ml | 25-150 | ng/ml | PO, oral; adults; patients; | The Pharmacological Basis of Therapeutics | |
| T Max | 2.5 | h | 1-4 | h | PO, oral; adults; patients; | The Pharmacological Basis of Therapeutics | |
| Metabolic | 20.0 | % | 20 | % | Urinary excretion; Unchanged drug; | DRUGBANK | Metabolic | 37.0 | % | 37 | % | Urinary excretion; | DRUGBANK |
| Clearance | 0.52 | L/h/kg | 8.6±2.9 | ml/min/kg | apparent clearance; IM,intramuscular injection; | Children ↓ ;Hepatitis, Hep → ; | The Pharmacological Basis of Therapeutics | Clearance | 0.96 | L/h/kg | 16 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 20.0 | L/kg | 20.0 | L/kg | DRUGBANK | Volume of Distribution | 21.0 | L/kg | 21±9 | L/kg | IM,intramuscular injection; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 10.0 | L/kg | 10 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 30.0 | h | ~30 | h | DRUGBANK | Half-life | 30.0 | h | 30±7 | h | The Pharmacological Basis of Therapeutics | Half-life | 25.0 | h | 25±15 | h | terminal half-life; IM,intramuscular injection; | The Pharmacological Basis of Therapeutics | Half-life | 11.0 | h | 11 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 37.0 | % | ~37 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 1.0 | % | <1 | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 90.0 | % | >90 | % | plasma proteins; | DRUGBANK | Protein Binding | 96.5 | % | 95-98 | % | RD, renal impairment, Renal disease,including uremia → ; | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 50.0 | mg/day | 50 | mg/day | PO, oral | Chlorpromazine Hydrochloride Injection | chlorpromazine hydrochloride | PDR |
| Max dose for infants | 40.0 | mg/day | 40 | mg/day | IM,intramuscular injection | Chlorpromazine Hydrochloride Injection | chlorpromazine hydrochloride | PDR |
| Max dose for children | 75.0 | mg/day | 75 | mg/day | IM,intramuscular injection | Chlorpromazine Hydrochloride Injection | chlorpromazine hydrochloride | PDR |
| Max dose for children | 50.0 | mg/day | 50 | mg/day | PO, oral | Chlorpromazine Hydrochloride Injection | chlorpromazine hydrochloride | PDR |
| Max dose for children | 40.0 | mg/day | 40 | mg/day | IM,intramuscular injection | Chlorpromazine Hydrochloride Injection | chlorpromazine hydrochloride | PDR |
| Max dose for children | 150.0 | mg/day | 150 | mg/day | PO, oral | Chlorpromazine Hydrochloride Injection | chlorpromazine hydrochloride | PDR |
| Max dose for adolescents | 1000.0 | mg/day | 1000 | mg/day | PO, oral | Chlorpromazine Hydrochloride Injection | chlorpromazine hydrochloride | PDR |
| Max dose for adolescents | 2000.0 | mg/day | 2000 | mg/day | PO, oral | Chlorpromazine Hydrochloride Injection | chlorpromazine hydrochloride | PDR |
| Max dose for adults | 1000.0 | mg/day | 1000 | mg/day | PO, oral | Chlorpromazine Hydrochloride Injection | chlorpromazine hydrochloride | PDR |
| Max dose for adults | 2000.0 | mg/day | 2000 | mg/day | PO, oral | Chlorpromazine Hydrochloride Injection | chlorpromazine hydrochloride | PDR |
| Max dose for elderly | 1000.0 | mg/day | 1000 | mg/day | PO, oral | Chlorpromazine Hydrochloride Injection | chlorpromazine hydrochloride | PDR |