Basic Information
| Drug ID | DDPD00476 |

|
| Drug Name | Duloxetine | |
| Molecular Weight | 297.415 | |
| Molecular Formula | C18H19NOS | |
| CAS Number | 116539-59-4 | |
| SMILES | CNCC[C@H](OC1=CC=CC2=CC=CC=C12)C1=CC=CS1 | |
| External Links | ||
| DRUGBANK | DB00476 | |
| T3DB | T3D2805 | |
| PubChem Compound | 60835 | |
| PDR | 288 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
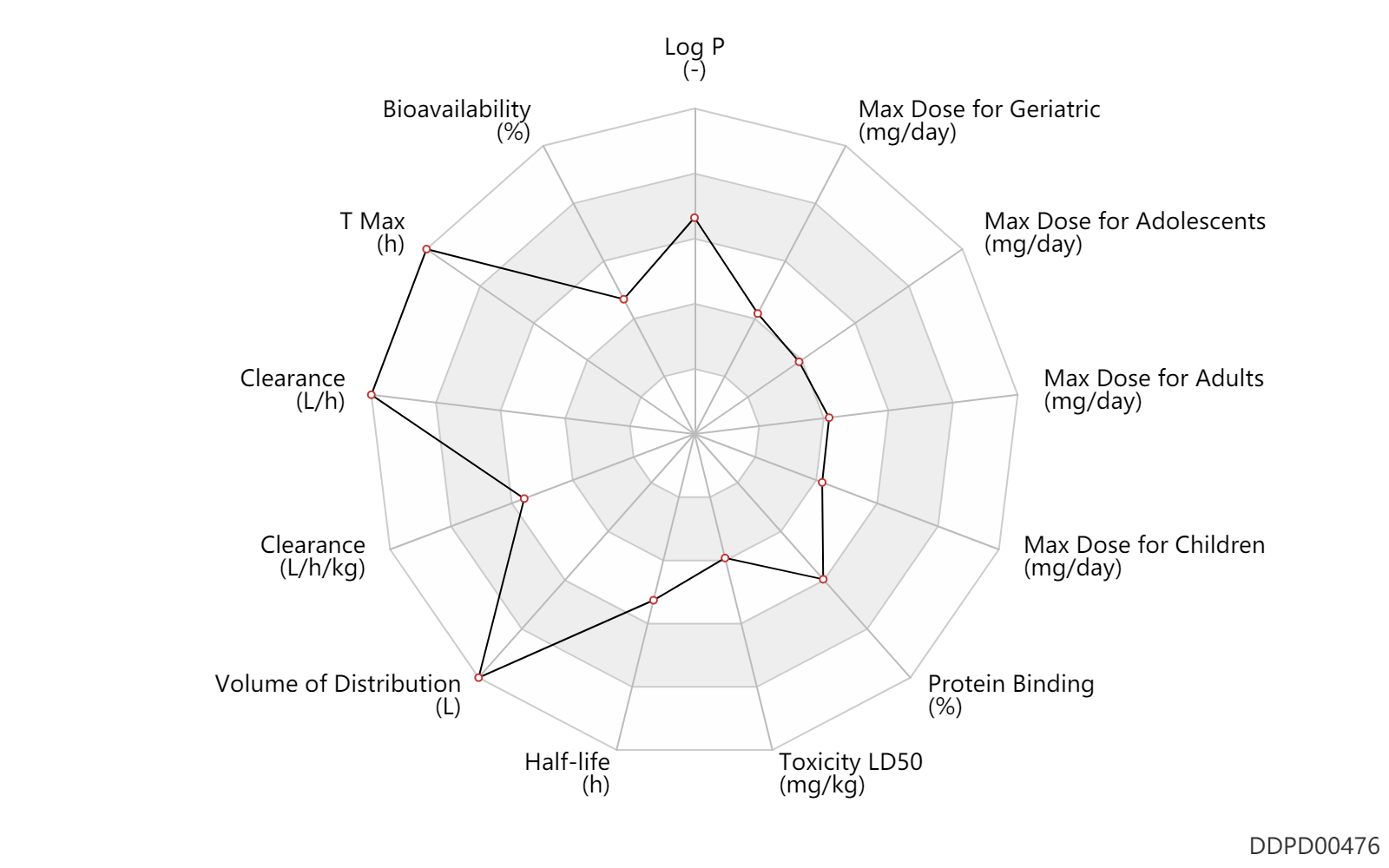
| Log P | 4.0 | - | 4.0 | - | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 50.0 | % | 50(30-80) | % | PO, oral; | DRUGBANK | |
| T Max | 6.0 | h | 6 | h | PO, oral; | DRUGBANK | T Max | 9.0 | h | 9 | h | food; | food ↑ ; | DRUGBANK |
| Clearance | 85.5 | L/h | 57-114 | L/h | DRUGBANK | Clearance | 0.51 | L/h/kg | 8.57 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 1710.0 | L | 1620-1800 | L | Apparent volume of distribution; | DRUGBANK | |
| Half-life | 12.0 | h | 12(8-17) | h | DRUGBANK | Half-life | 12.0 | h | 12 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 491.0 | mg/kg | 491.0 | mg/kg | PO, oral; male rat; | T3DB | Toxicity LD50 | 279.0 | mg/kg | 279.0 | mg/kg | PO, oral; female rat; | T3DB |
| Protein Binding | 90.0 | % | >90 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 120.0 | mg/day | 120 | mg/day | PO, oral | Cymbalta | duloxetine | PDR |
| Max dose for adolescents | 120.0 | mg/day | 120 | mg/day | PO, oral | Cymbalta | duloxetine | PDR |
| Max dose for adolescents | 60.0 | mg/day | 60 | mg/day | PO, oral | Cymbalta | duloxetine | PDR |
| Max dose for adults | 120.0 | mg/day | 120 | mg/day | PO, oral | Cymbalta | duloxetine | PDR |
| Max dose for geriatric | 120.0 | mg/day | 120 | mg/day | PO, oral | Cymbalta | duloxetine | PDR |