Basic Information
| Drug ID | DDPD00446 |

|
| Drug Name | Chloramphenicol | |
| Molecular Weight | 323.129 | |
| Molecular Formula | C11H12Cl2N2O5 | |
| CAS Number | 56-75-7 | |
| SMILES | OC[C@@H](NC(=O)C(Cl)Cl)[C@H](O)C1=CC=C(C=C1)[N+]([O-])=O | |
| External Links | ||
| DRUGBANK | DB00446 | |
| T3DB | T3D3954 | |
| PubChem Compound | 5959 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
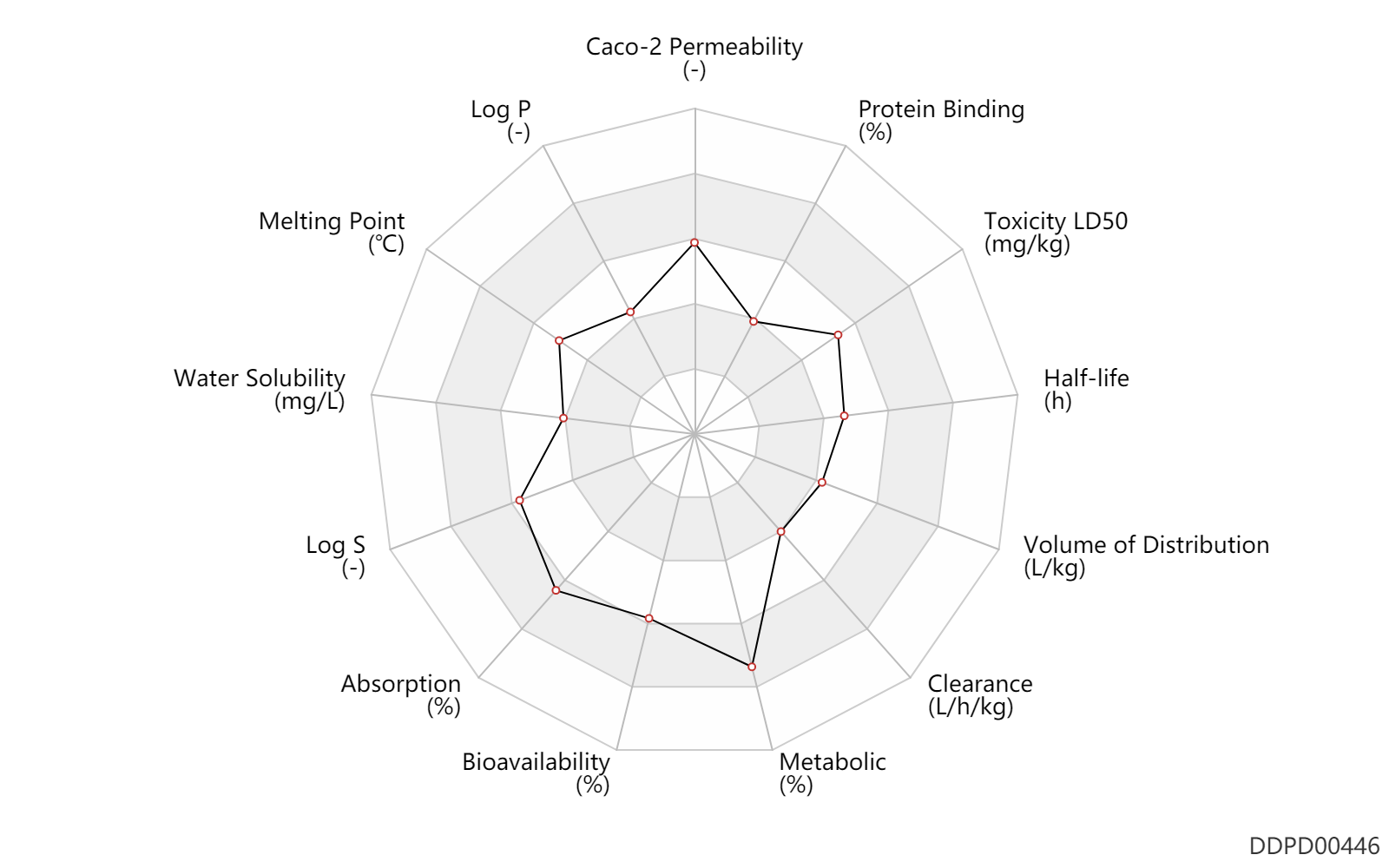
| Caco-2 Permeability | -4.69 | - | -4.69 | - | ADME Research, USCD |
| Log P | 1.14 | - | 1.14 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 171.0 | ℃ | 171 | ℃ | Bartz, Q.R.; U.S. Patent 2,483,871; October 4, 1949; assigned to Parke, Davis & Company Crooks, H.M., Jr., Rebstock, M.C., Controulis, J. and Bartz, Q.R.; U.S. Patent 2,483,884; October 4, 1949; assigned to Parke, Davis & Company. Ehrlich, J., Smith, R.M. and Penner, M.A.; U.S. Patent 2,483,892; October 4, 1949; assigned to Parke, Davis & Company. Carrara, G.; U.S. Patent 2,776,312; January 1, 1957 Slack, R.; U.S. Patent 2,786,870; March 26, 1957; assigned to Parke, Davis & Company. |
| Water Solubility | 2500.0 | mg/L | 2500 | mg/L | MERCK INDEX 2001) |
| Log S | -2.11 | - | -2.11 | - | ADME Research, USCD |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Absorption | 100.0 | % | 100.0 | % | PO, oral; | DRUGBANK | |
| Bioavailability | 80.0 | % | 80.0 | % | PO, oral; | DRUGBANK | Bioavailability | 70.0 | % | 70.0 | % | IM,intramuscular injection; | DRUGBANK |
| Metabolic | 90.0 | % | 90 | % | Liver metabolism; Inactive metabolite; | DRUGBANK | |
| Clearance | 0.14 | L/h/kg | 2.4 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset | |
| Volume of Distribution | 0.94 | L/kg | 0.94 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset | |
| Half-life | 2.5 | h | 1.5-3.5 | h | normal renal function; normal hepatic function; adults; | DRUGBANK | Half-life | 3.5 | h | 3-4 | h | RD, renal impairment, Renal disease,including uremia; patients; | DRUGBANK | Half-life | 8.1 | h | 4.6-11.6 | h | severe hepatic impairment; patients; | DRUGBANK | Half-life | 4.8 | h | 3-6.5 | h | Infants; Children; | DRUGBANK | Half-life | 24.0 | h | >=24 | h | Neonates; | gaining weight ↑ ; | DRUGBANK | Half-life | 4.6 | h | 4.6 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 1500.0 | mg/kg | 1500.0 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 2500.0 | mg/kg | 2500.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 1500.0 | mg/kg | 1500.0 | mg/kg | PO, oral; mouse; | T3DB | Toxicity LD50 | 2500.0 | mg/kg | 2500.0 | mg/kg | PO, oral; Rattus, Rat; | T3DB |
| Protein Binding | 55.0 | % | 50-60 | % | plasma proteins; adults; human, homo sapiens; | DRUGBANK | Protein Binding | 32.0 | % | 32 | % | plasma proteins; Prem, premature; human, homo sapiens; | DRUGBANK |
Maximum Dosage
Not Available