Basic Information
| Drug ID | DDPD00438 |

|
| Drug Name | Ceftazidime | |
| Molecular Weight | 546.576 | |
| Molecular Formula | C22H22N6O7S2 | |
| CAS Number | 72558-82-8 | |
| SMILES | [O-]C(=O)C1=C(CS[C@]2([H])[C@H](NC(=O)C(=N/OC(C)(C)C(O)=O)\C3=CSC(N)=N3)C(=O)N12)C[N+]1=CC=CC=C1 | |
| External Links | ||
| DRUGBANK | DB00438 | |
| PubChem Compound | 5481173 | |
| PDR | 1607 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
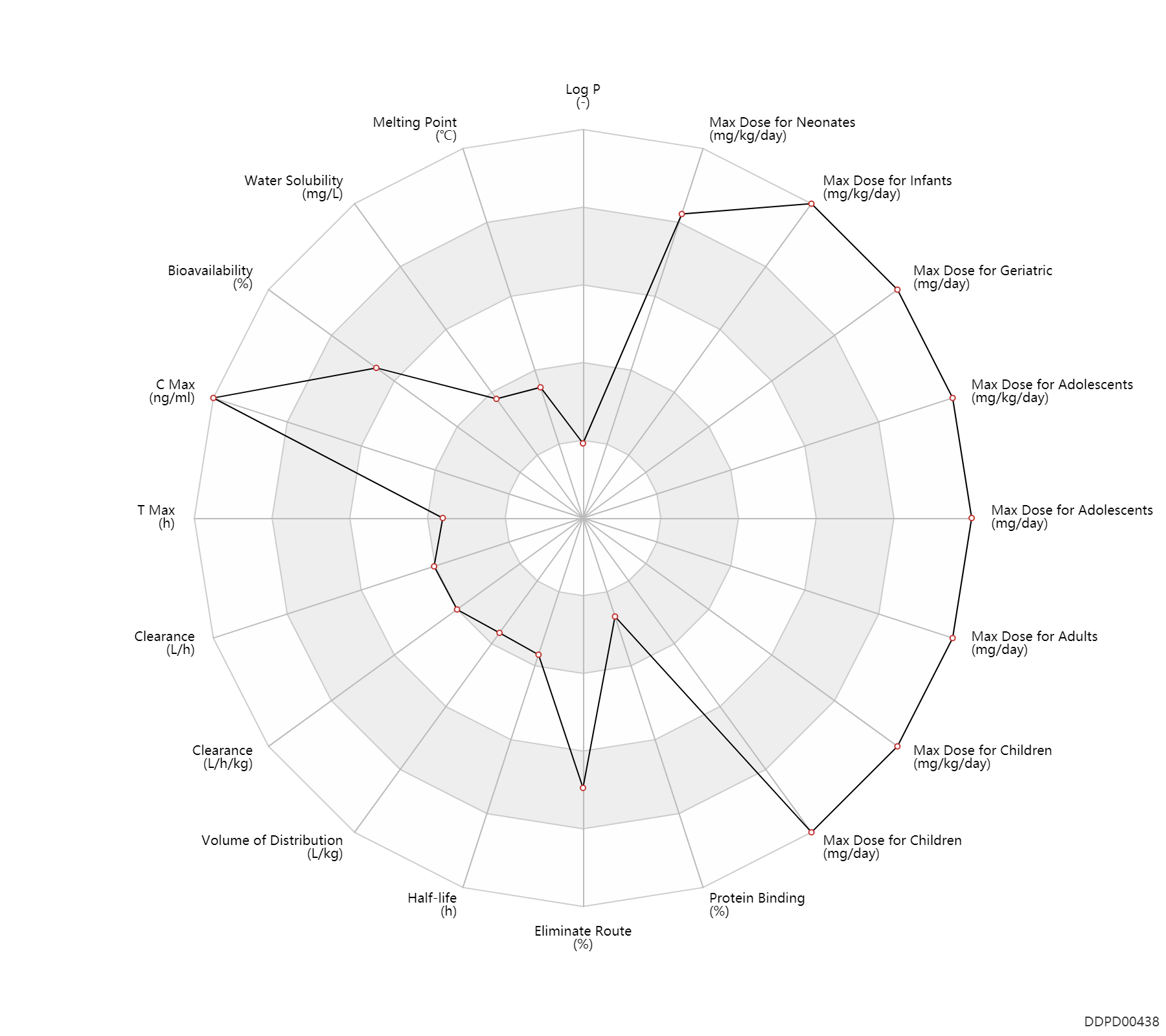
| Log P | -1.6 | - | -1.6 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 108.0 | ℃ | 103-113 | ℃ | O'Callaghan, C.H., Livermore, D.G.H. and Newall, C.E.; British Patent 2,025,398; January 23, 1980; assigned to Glaxo Group Ltd. |
| Water Solubility | 396.0 | mg/L | 396 | mg/L | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 91.0 | % | 91.0 | % | IM,intramuscular injection; | The Pharmacological Basis of Therapeutics | |
| C Max | 34000.0 | ng/ml | 29-39 | mcg/ml | IM,intramuscular injection; adults; normal,healthy; | The Pharmacological Basis of Therapeutics | C Max | 132500.0 | ng/ml | 119-146 | mcg/ml | intravenous injection, IV; adults; normal,healthy; | The Pharmacological Basis of Therapeutics |
| T Max | 1.0 | h | 0.7-1.3 | h | IM,intramuscular injection; adults; normal,healthy; | The Pharmacological Basis of Therapeutics | |
| Clearance | 6.9 | L/h | 115.0 | ml/min | DRUGBANK | Clearance | 0.14 | L/h/kg | 2.4 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 0.23 | L/kg | 0.23±0.02 | L/kg | Elderly ↑ ;RD, renal impairment, Renal disease,including uremia → ;Cystic fibrosis → ;Burn ↑ ; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 0.31 | L/kg | 0.31 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 1.9 | h | ~1.9 | h | intravenous injection, IV; | RD, renal impairment, Renal disease,including uremia ↑ ; | DRUGBANK | Half-life | 1.6 | h | 1.6±0.1 | h | Prem, premature ↑ ;Neonates ↑ ;RD, renal impairment, Renal disease,including uremia ↑ ;Cystic fibrosis → ;Age ↑ ; | The Pharmacological Basis of Therapeutics | Half-life | 1.8 | h | 1.8 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 84.0 | % | 84±4 | % | Urinary excretion; Unchanged drug; | Cystic fibrosis → ; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 10.0 | % | <10 | % | DRUGBANK | Protein Binding | 21.0 | % | 21±6 | % | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 60.0 | mg/kg/day | 60 | mg/kg/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for neonates | 150.0 | mg/kg/day | 150 | mg/kg/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for neonates | 60.0 | mg/kg/day | 60 | mg/kg/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for neonates | 100.0 | mg/kg/day | 100 | mg/kg/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for neonates | 150.0 | mg/kg/day | 150 | mg/kg/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for infants | 150.0 | mg/kg/day | 150 | mg/kg/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for infants | 400.0 | mg/kg/day | 400 | mg/kg/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for children | 150.0 | mg/kg/day | 150 | mg/kg/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for children | 6000.0 | mg/day | 6 | g/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for children | 400.0 | mg/kg/day | 400 | mg/kg/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for children | 12000.0 | mg/day | 12 | g/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for adolescents | 6000.0 | mg/day | 6 | g/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for adolescents | 400.0 | mg/kg/day | 400 | mg/kg/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for adolescents | 12000.0 | mg/day | 12 | g/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for adults | 6000.0 | mg/day | 6 | g/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for adults | 12000.0 | mg/day | 12 | g/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for geriatric | 6000.0 | mg/day | 6 | g/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |
| Max dose for geriatric | 12000.0 | mg/day | 12 | g/day | intravenous injection, IV;IM,intramuscular injection; | Tazicef | ceftazidime | PDR |