Basic Information
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
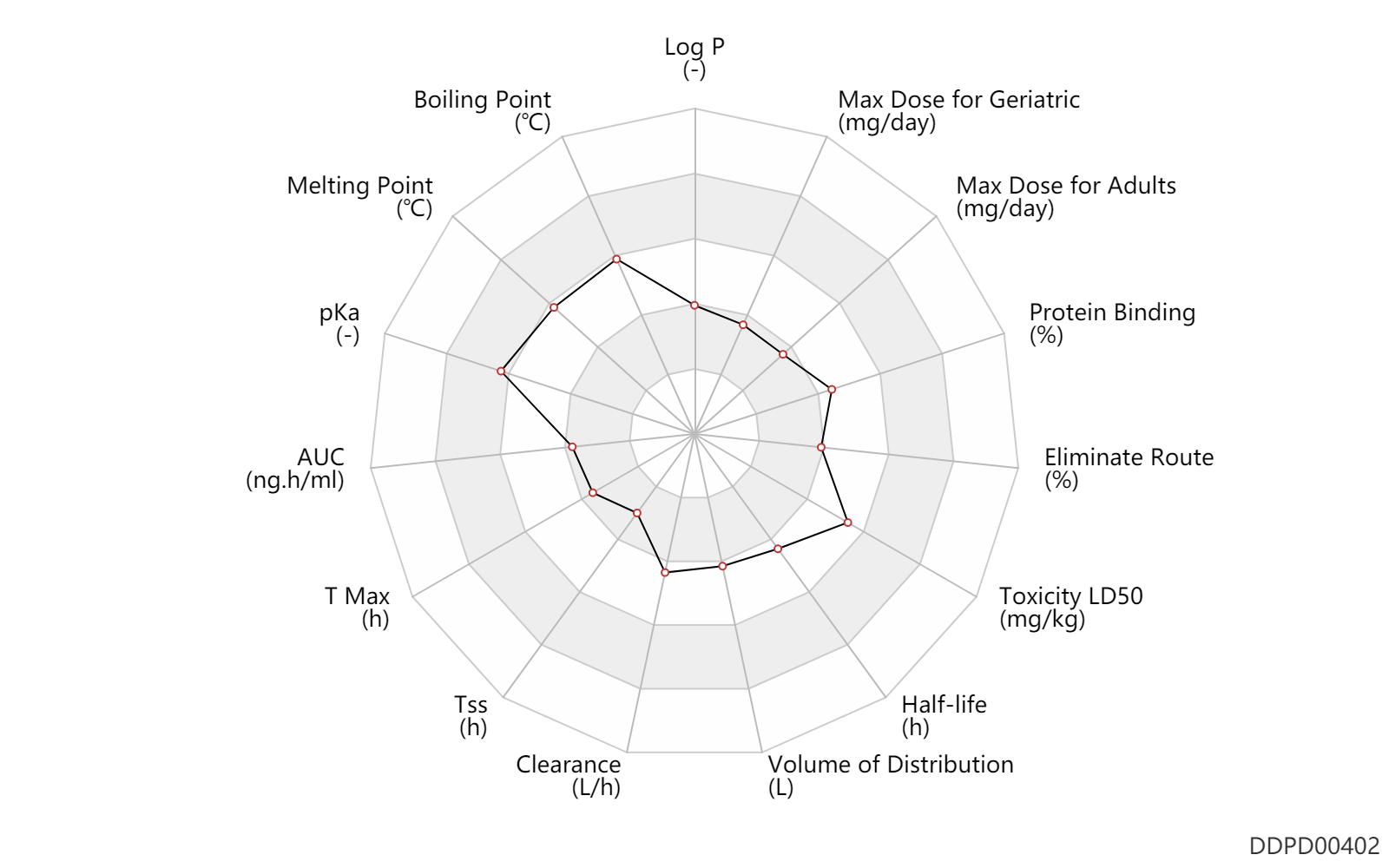
| Log P | 0.81 | - | 0.81 | - | http://www.t3db.ca/toxins/T3D4552 |
| Boiling Point | 487.2 | ℃ | 487.2 | ℃ | https://www.lookchem.com/Timolol/ |
| Melting Point | 202.5 | ℃ | 202-203 | ℃ | https://www.chemicalbook.com/ChemicalProductProperty_EN_CB3711352.htm |
| pKa | 9.2 | - | 9.2 | - | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| AUC | 278.0 | ng.h/ml | 278.0 | ng.h/ml | PO, oral; | DRUGBANK | |
| T Max | 1.0 | h | 1 | h | PO, oral; | DRUGBANK | |
| Tss | 36.0 | h | 24-48 | h | PO, oral; | DRUGBANK | |
| Clearance | 11.0 | L/h | 184.0 | ml/min | Average clearance; normal,healthy; young; | DRUGBANK | |
| Volume of Distribution | 89.9 | L | 89.9 | L | DRUGBANK | ||
| Half-life | 6.1 | h | 6.1 | h | normal,healthy; patients; | hepatopathy,LD ↑ ;Elderly ↑ ; | DRUGBANK |
| Toxicity LD50 | 980.0 | mg/kg | 980.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 3200.0 | mg/kg | 3200.0 | mg/kg | PO, oral; rabbit; | DRUGBANK |
| Eliminate Route | 10.0 | % | ~10 | % | Urinary excretion; | DRUGBANK | |
| Protein Binding | 55.5 | % | 52-59 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 3.0 | mg/day | 3 | mg/day | PO, oral | Lunesta | eszopiclone | PDR |
| Max dose for geriatric | 2.0 | mg/day | 2 | mg/day | PO, oral | Lunesta | eszopiclone | PDR |