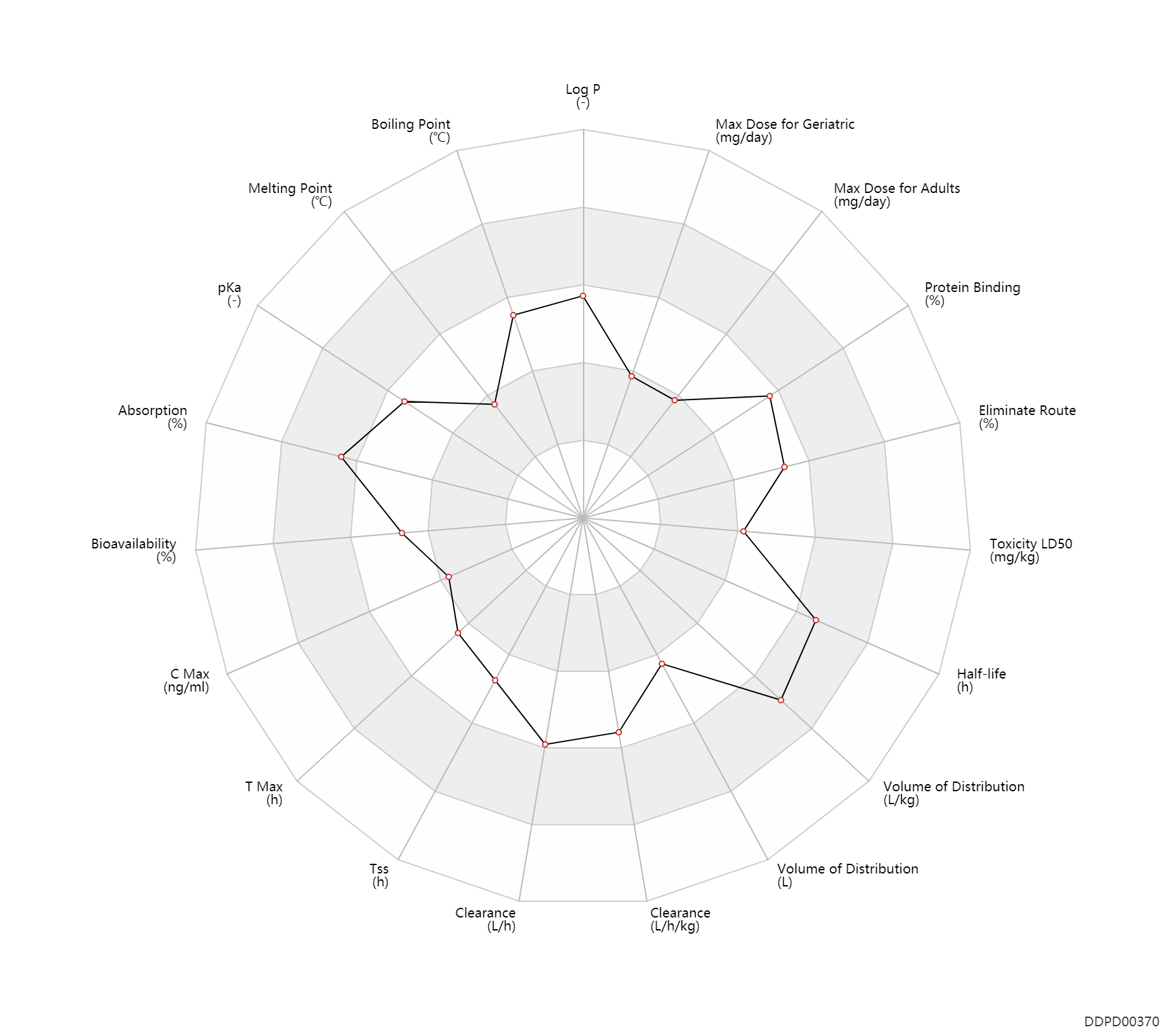
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| Absorption |
100.0 |
% |
100.0 |
% |
PO, oral; food; |
food → ; |
DRUGBANK |
| Bioavailability |
50.0 |
% |
50.0 |
% |
PO, oral; food; |
food → ; |
DRUGBANK |
Bioavailability |
50.0 |
% |
50±10 |
% |
PO, oral; |
|
The Pharmacological Basis of Therapeutics |
| C Max |
41.8 |
ng/ml |
41.8±7.7 |
ng/ml |
PO, oral; |
|
The Pharmacological Basis of Therapeutics |
| T Max |
2.0 |
h |
2 |
h |
PO, oral; food; |
food → ; |
DRUGBANK |
T Max |
1.5 |
h |
1.5±0.7 |
h |
PO, oral; |
|
The Pharmacological Basis of Therapeutics |
| Tss |
120.0 |
h |
5.0 |
day |
PO, oral; |
|
DRUGBANK |
| Clearance |
31.0 |
L/h |
31.0 |
L/h |
Total clearance; intravenous injection, IV; Male, men; |
hepatopathy,LD ↓ ;RD, renal impairment, Renal disease,including uremia ↓ ;Age ↓ ; |
DRUGBANK |
Clearance |
0.55 |
L/h/kg |
9.12±1.14 |
ml/min/kg |
normal,healthy; adults; hydrolysis; hydrolysis; hydrolysis; |
hepatopathy,LD ↓ ;moderate renal function ↓ ;severe renal function ↓ ; |
The Pharmacological Basis of Therapeutics |
Clearance |
0.48 |
L/h/kg |
8 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
107.0 |
L |
107±42 |
L |
PO, oral; |
|
DRUGBANK |
Volume of Distribution |
4.5 |
L/kg |
4.5±1.7 |
L/kg |
normal,healthy; adults; hydrolysis; hydrolysis; hydrolysis; |
|
The Pharmacological Basis of Therapeutics |
Volume of Distribution |
4.2 |
L/kg |
4.2 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
30.0 |
h |
20-40 |
h |
|
|
DRUGBANK |
Half-life |
16.3 |
h |
16.3±4.6 |
h |
|
chronic liver disease ↑ ;moderate renal function ↑ ;severe renal function ↑ ; |
The Pharmacological Basis of Therapeutics |
Half-life |
15.0 |
h |
15 |
h |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 |
830.0 |
mg/kg |
830.0 |
mg/kg |
PO, oral; Male, men; mouse; |
|
DRUGBANK |
Toxicity LD50 |
660.0 |
mg/kg |
600-720 |
mg/kg |
PO, oral; mouse; |
|
T3DB |
Toxicity LD50 |
405.0 |
mg/kg |
320-490 |
mg/kg |
PO, oral; Rattus, Rat; |
|
T3DB |
| Eliminate Route |
75.0 |
% |
75 |
% |
Urinary excretion; |
|
DRUGBANK |
Eliminate Route |
15.0 |
% |
15 |
% |
Faeces excretion; |
|
DRUGBANK |
| Protein Binding |
85.0 |
% |
~85 |
% |
plasma proteins; |
|
DRUGBANK |
Protein Binding |
85.0 |
% |
85 |
% |
adults; normal,healthy; human, homo sapiens; |
|
The Pharmacological Basis of Therapeutics |