Basic Information
| Drug ID | DDPD00349 |

|
| Drug Name | Clobazam | |
| Molecular Weight | 300.74 | |
| Molecular Formula | C16H13ClN2O2 | |
| CAS Number | 22316-47-8 | |
| SMILES | CN1C2=C(C=C(Cl)C=C2)N(C2=CC=CC=C2)C(=O)CC1=O | |
| External Links | ||
| DRUGBANK | DB00349 | |
| T3DB | T3D4564 | |
| PubChem Compound | 2789 | |
| PDR | 24262 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
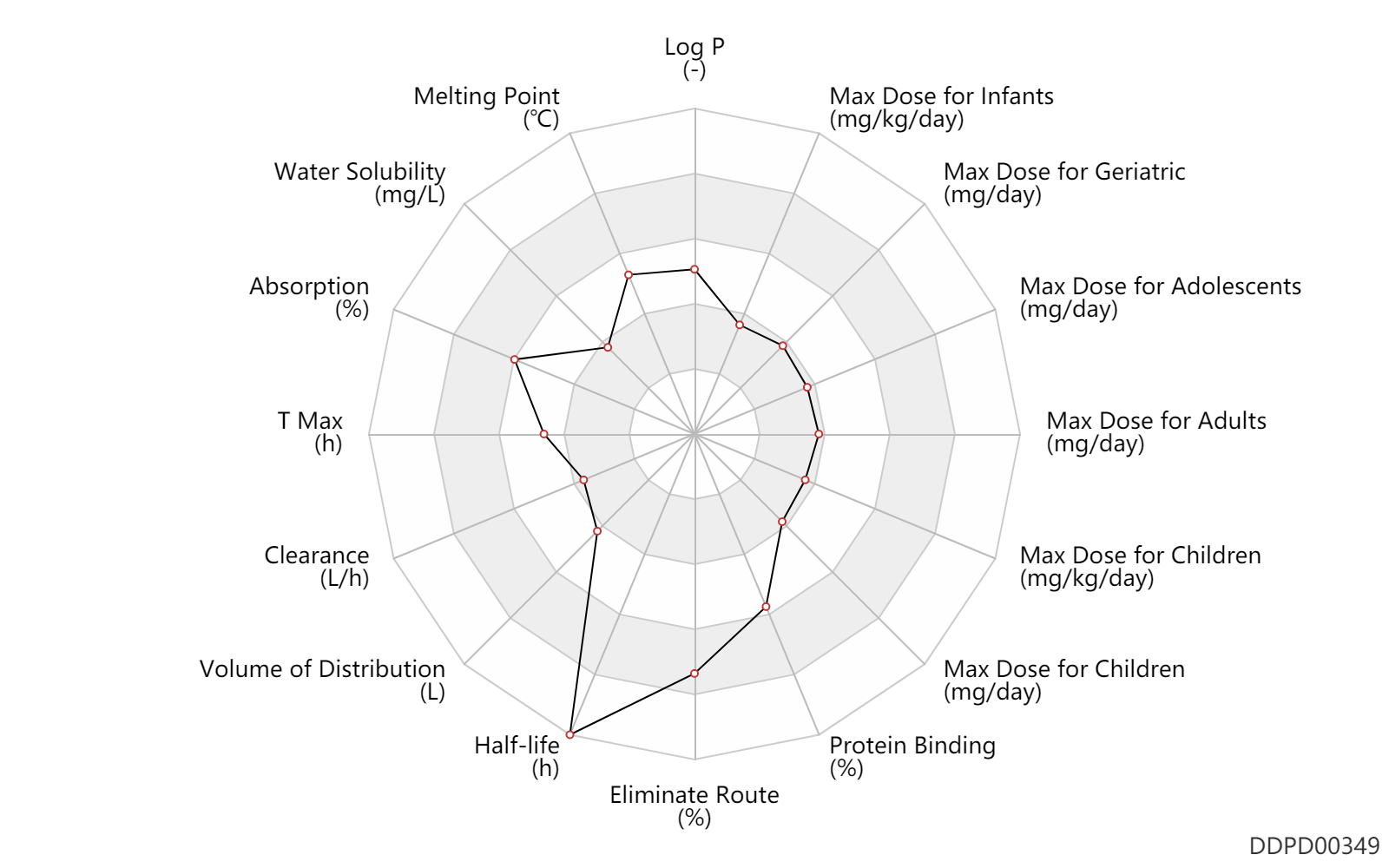
| Log P | 2.12 | - | 2.12 | - | HENCZI,M ET AL. (1995) |
| Melting Point | 181.0 | ℃ | 180-182 | ℃ | Hauptmann, K.H., Weber, K.-H., Zeile, K., Danneberg, P. and Giesemann, R.; South African Patent 68/0803; February 7,1968; assigned to Boehringer lngelheim GmbH, Germany. |
| Water Solubility | 188.0 | mg/L | 188 | mg/L | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Absorption | 87.0 | % | 87.0 | % | PO, oral; food; | food → ; | DRUGBANK |
| T Max | 2.0 | h | 1-3 | h | PO, oral; | DRUGBANK | |
| Clearance | 2.5 | L/h | ~2.49 | L/h | DRUGBANK | ||
| Volume of Distribution | 100.0 | L | 100.0 | L | DRUGBANK | ||
| Half-life | 32.0 | h | 32 | h | elimination half-life; PO, oral; | DRUGBANK | Half-life | 57.0 | h | 57 | h | epilepsy ↑ ; | DRUGBANK |
| Eliminate Route | 94.0 | % | ~94 | % | Urinary excretion; | DRUGBANK | |
| Protein Binding | 85.0 | % | 80-90 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 2.0 | mg/kg/day | 2 | mg/kg/day | PO, oral | Sympazan | clobazam | PDR |
| Max dose for children | 40.0 | mg/day | 40 | mg/day | PO, oral | Sympazan | clobazam | PDR |
| Max dose for children | 20.0 | mg/day | 20 | mg/day | PO, oral | Sympazan | clobazam | PDR |
| Max dose for children | 2.0 | mg/kg/day | 2 | mg/kg/day | PO, oral | Sympazan | clobazam | PDR |
| Max dose for adolescents | 40.0 | mg/day | 40 | mg/day | PO, oral | Sympazan | clobazam | PDR |
| Max dose for adolescents | 20.0 | mg/day | 20 | mg/day | PO, oral | Sympazan | clobazam | PDR |
| Max dose for adults | 40.0 | mg/day | 40 | mg/day | PO, oral | Sympazan | clobazam | PDR |
| Max dose for geriatric | 40.0 | mg/day | 40 | mg/day | PO, oral | Sympazan | clobazam | PDR |