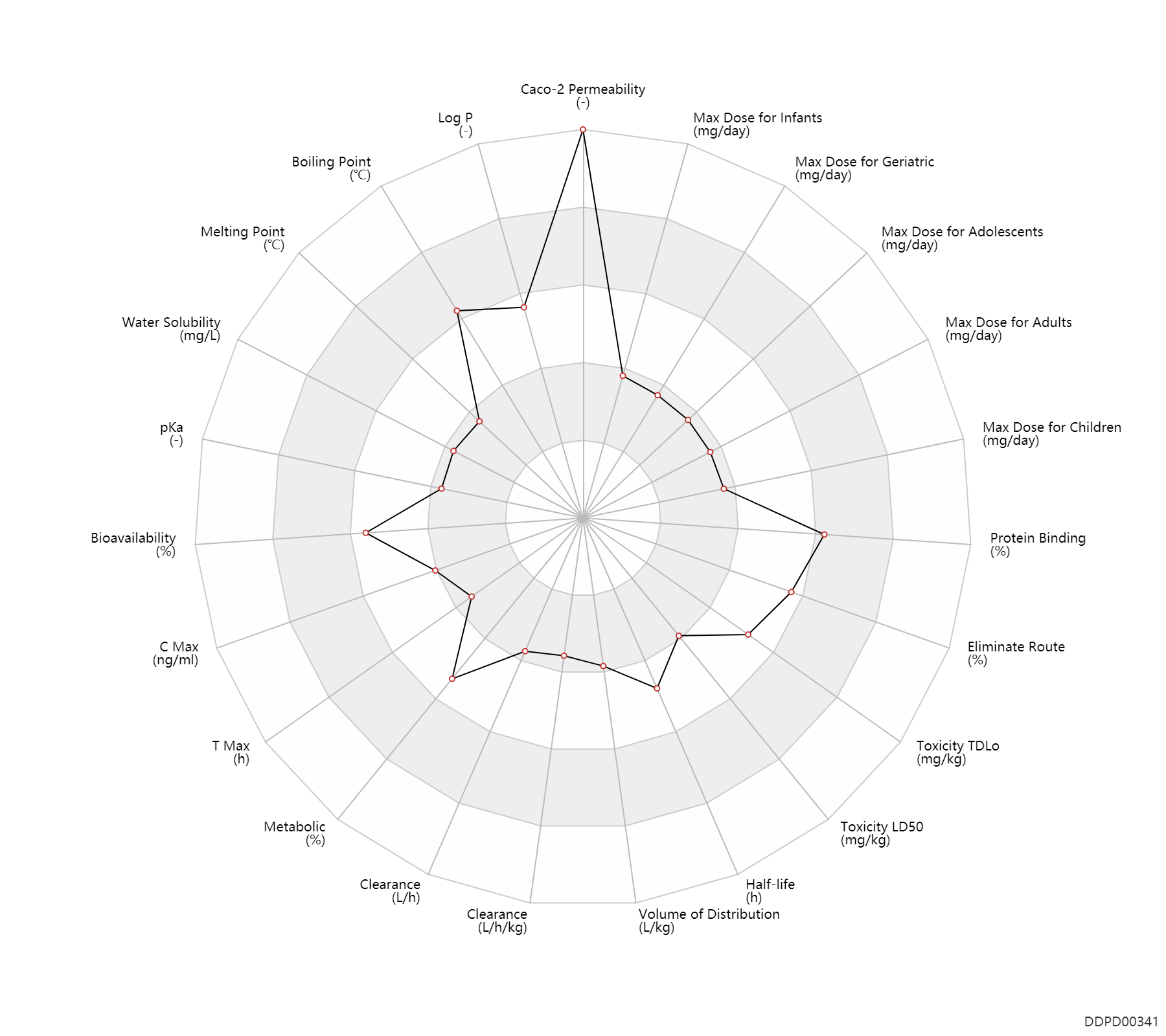
Basic Information
| Drug ID | DDPD00341 |

|
| Drug Name | Cetirizine | |
| Molecular Weight | 388.888 | |
| Molecular Formula | C21H25ClN2O3 | |
| CAS Number | 83881-51-0 | |
| SMILES | OC(=O)COCCN1CCN(CC1)C(C1=CC=CC=C1)C1=CC=C(Cl)C=C1 | |
| External Links | ||
| DRUGBANK | DB00341 | |
| T3DB | T3D2756 | |
| PubChem Compound | 2678 | |
| PDR | 2934 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Caco-2 Permeability | 1.14 | - | 1.14 | - | https://core.ac.uk/download/pdf/76975249.pdf |
| Log P | 2.8 | - | 2.8 | - | http://www.t3db.ca/toxins/T3D2756 |
| Boiling Point | 542.1 | ℃ | 542.1 | ℃ | https://www.lookchem.com/Cetirizine-hydrochloride/ |
| Melting Point | 112.5 | ℃ | 110-115 | ℃ | https://www.chemicalbook.com/ChemicalProductProperty_US_CB3440625.aspx |
| Water Solubility | 101.0 | mg/L | 101 | mg/L | https://www.chemicalbook.com/ChemicalProductProperty_US_CB3440625.aspx |
| pKa | 1.52 | - | 1.52,2.92,8.27 | - | https://www.researchgate.net/publication/42768947_Isolation_and_Characterization_of_Cetirizine_Degradation_Product_Mechanism_of_Cetirizine_Oxidation | pKa | 2.92 | - | 1.52,2.92,8.27 | - | https://www.researchgate.net/publication/42768947_Isolation_and_Characterization_of_Cetirizine_Degradation_Product_Mechanism_of_Cetirizine_Oxidation | pKa | 8.27 | - | 1.52,2.92,8.27 | - | https://www.researchgate.net/publication/42768947_Isolation_and_Characterization_of_Cetirizine_Degradation_Product_Mechanism_of_Cetirizine_Oxidation |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 70.0 | % | >70 | % | The Pharmacological Basis of Therapeutics | ||
| C Max | 311.0 | ng/ml | 311.0 | ng/ml | Tablet, PO, oral; Capsule, PO, Oral; normal,healthy; | DRUGBANK | C Max | 313.0 | ng/ml | 313±45 | ng/ml | Oral single dose; normal,healthy; | The Pharmacological Basis of Therapeutics |
| T Max | 0.90 | h | 0.9±0.2 | h | Oral single dose; normal,healthy; | The Pharmacological Basis of Therapeutics | |
| Metabolic | 70.0 | % | 70 | % | Urinary excretion; | DRUGBANK | Metabolic | 10.0 | % | 10 | % | Faeces excretion; | DRUGBANK |
| Clearance | 3.2 | L/h | ~53 | ml/min | Total clearance; Renal metabolism; | DRUGBANK | Clearance | 0.0444 | L/h/kg | 0.74±0.19 | ml/min/kg | apparent clearance; normal,healthy; Male, men; Female, women; | Children ↑ ;Elderly ↓ ;hepatopathy,LD ↓ ;RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics |
| Volume of Distribution | 0.44 | L/kg | 0.44±0.19 | L/kg | Apparent volume of distribution; | DRUGBANK | Volume of Distribution | 0.58 | L/kg | 0.58±0.16 | L/kg | Apparent volume of distribution; normal,healthy; Male, men; Female, women; | The Pharmacological Basis of Therapeutics |
| Half-life | 8.3 | h | 8.3 | h | elimination half-life; | DRUGBANK | Half-life | 9.4 | h | 9.42±2.4 | h | normal,healthy; Male, men; Female, women; | Children ↓ ;chronic liver disease ↑ ;RD, renal impairment, Renal disease,including uremia ↑ ;Age ↑ ; | The Pharmacological Basis of Therapeutics |
| Toxicity LD50 | 365.0 | mg/kg | 365.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | |
| Toxicity TDLo | 50.0 | mg/kg | 50.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity TDLo | 0.10 | mg/kg | 0.1 | mg/kg | PO, oral; mouse; | DRUGBANK |
| Toxicity LDLo | 138.0 | mg/kg | 138.0 | mg/kg | Intraperitoneal, IP; mouse; | DRUGBANK | |
| Eliminate Route | 77.5 | % | 70-85 | % | Urinary excretion; PO, oral; | DRUGBANK | Eliminate Route | 11.5 | % | 10-13 | % | Faeces excretion; PO, oral; | DRUGBANK | Eliminate Route | 70.9 | % | 70.9±7.8 | % | Urinary excretion; normal,healthy; human, homo sapiens; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 93.0 | % | 93 | % | plasma proteins; | DRUGBANK | Protein Binding | 98.8 | % | 98.8±0.8 | % | normal,healthy; human, homo sapiens; | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 2.5 | mg/day | 2.5 | mg/day | PO, oral;intravenous injection, IV; | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for children | 10.0 | mg/day | 10 | mg/day | PO, oral;intravenous injection, IV; | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for children | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for children | 5.0 | mg/day | 5 | mg/day | PO, oral | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for children | 2.5 | mg/day | 2.5 | mg/day | intravenous injection, IV | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for children | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for children | 5.0 | mg/day | 5 | mg/day | PO, oral | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for children | 0.25 | mg/kg | 0.25 | mg/kg | PO, oral | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for children | 2.5 | mg/day | 2.5 | mg/day | intravenous injection, IV | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for children | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for children | 5.0 | mg/day | 5 | mg/day | PO, oral | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for children | 0.25 | mg/kg | 0.25 | mg/kg | PO, oral | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for children | 2.5 | mg/day | 2.5 | mg/day | intravenous injection, IV | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for adolescents | 10.0 | mg/day | 10 | mg/day | PO, oral;intravenous injection, IV; | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for adolescents | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for adults | 10.0 | mg/day | 10 | mg/day | PO, oral;intravenous injection, IV; | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for adults | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for geriatric | 10.0 | mg/day | 10 | mg/day | PO, oral;intravenous injection, IV; | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for geriatric | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for geriatric | 5.0 | mg/day | 5 | mg/day | PO, oral | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for geriatric | 10.0 | mg/day | 10 | mg/day | intravenous injection, IV | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |
| Max dose for geriatric | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Cetirizine Hydrochloride Tablets | cetirizine hydrochloride | PDR |