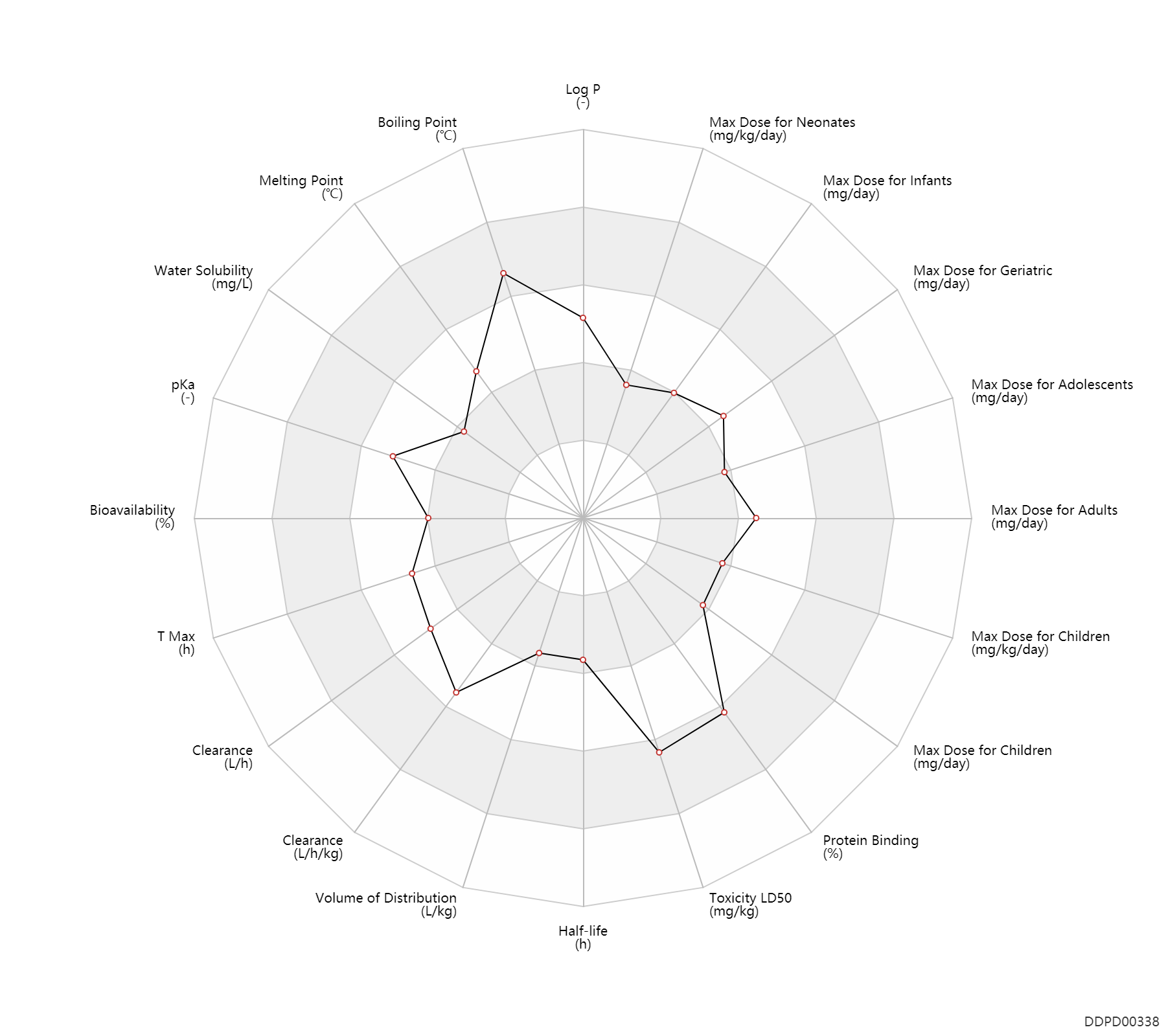
| Max dose for neonates |
0.7 |
mg/kg/day |
0.7 |
mg/kg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for neonates |
1.5 |
mg/kg/day |
1.5 |
mg/kg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for infants |
10.0 |
mg/day |
10 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for infants |
40.0 |
mg/day |
40 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for infants |
5.0 |
mg/day |
5 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for infants |
1.5 |
mg/day |
1.5 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for infants |
2.5 |
mg/day |
2.5 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for infants |
1.5 |
mg/day |
1.5 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for children |
20.0 |
mg/day |
20 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for children |
80.0 |
mg/day |
80 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for children |
10.0 |
mg/day |
10 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for children |
3.3 |
mg/kg/day |
3.3 |
mg/kg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for children |
40.0 |
mg/day |
40 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for children |
5.0 |
mg/day |
5 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for children |
3.3 |
mg/kg/day |
3.3 |
mg/kg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for adolescents |
40.0 |
mg/day |
40 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for adolescents |
80.0 |
mg/day |
80 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for adults |
40.0 |
mg/day |
40 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for adults |
160.0 |
mg/day |
160 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for adults |
360.0 |
mg/day |
360 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for geriatric |
40.0 |
mg/day |
40 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for geriatric |
160.0 |
mg/day |
160 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |
| Max dose for geriatric |
360.0 |
mg/day |
360 |
mg/day |
PO, oral |
Prilosec Delayed Release Capsules and Oral Suspension |
(omeprazole); (omeprazole magnesium) |
PDR |