Basic Information
| Drug ID | DDPD00337 |

|
| Drug Name | Pimecrolimus | |
| Molecular Weight | 810.46 | |
| Molecular Formula | C43H68ClNO11 | |
| CAS Number | 137071-32-0 | |
| SMILES | [H][C@]1(CC[C@H](Cl)[C@@H](C1)OC)\C=C(/C)[C@@]1([H])OC(=O)[C@]2([H])CCCCN2C(=O)C(=O)[C@]2(O)O[C@@]([H])([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC)C(=O)C[C@H](O)[C@H]1C)OC | |
| External Links | ||
| DRUGBANK | DB00337 | |
| PubChem Compound | 6509979 | |
| PDR | 2270 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
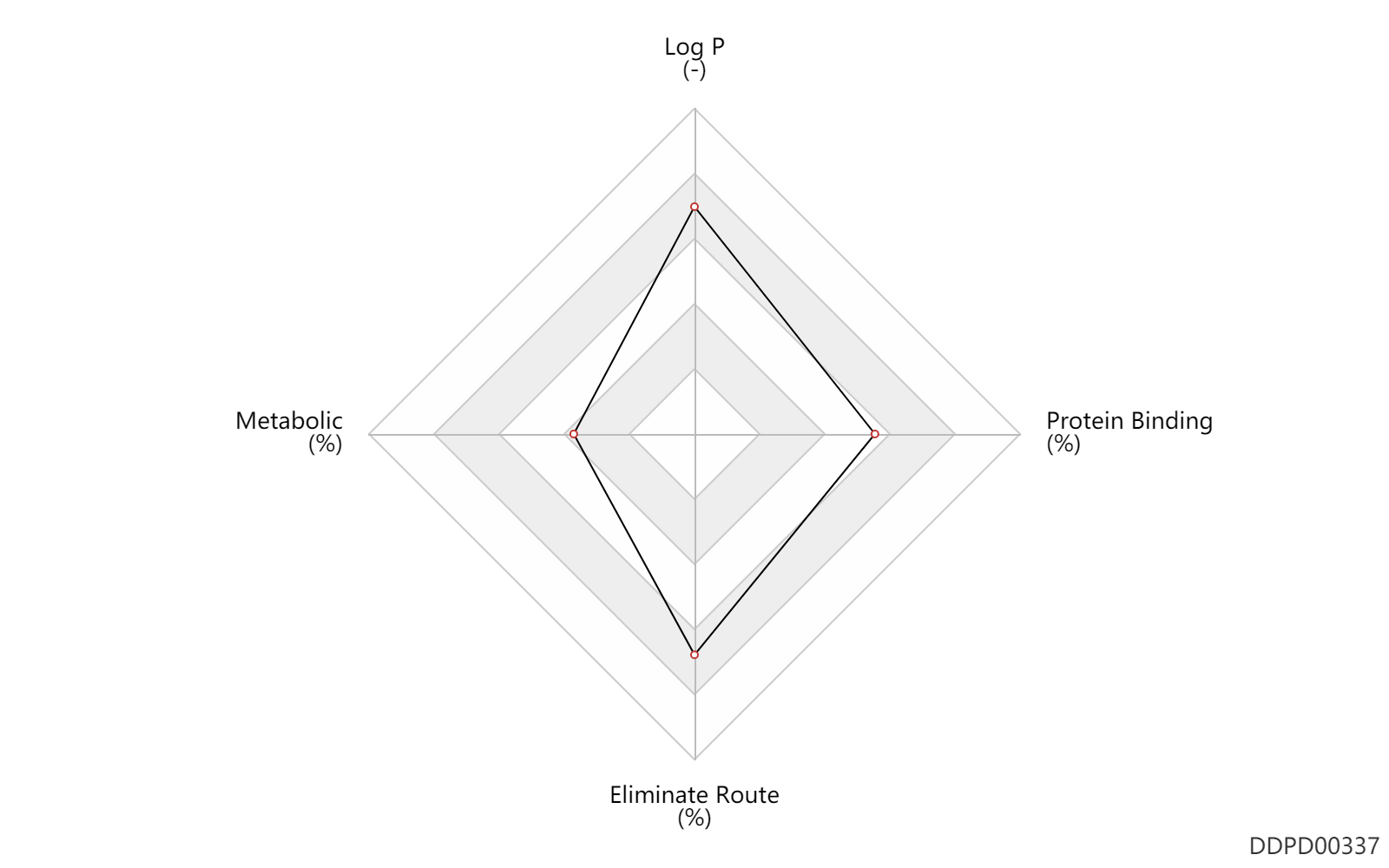
| Log P | 4.4 | - | 4.4 | - | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Metabolic | 0 | % | 0 | % | skin/dermal; | DRUGBANK |
| Eliminate Route | 80.0 | % | 80 | % | Faeces excretion; | DRUGBANK |
| Protein Binding | 80.5 | % | 74-87 | % | plasma proteins; high protein binding; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 2.0 | application/day | 2 | application/day | skin/dermal | Elidel | pimecrolimus | PDR |
| Max dose for adolescents | 2.0 | application/day | 2 | application/day | skin/dermal | Elidel | pimecrolimus | PDR |
| Max dose for adults | 2.0 | appLication/day | 2 | appLication/day | skin/dermal | Elidel | pimecrolimus | PDR |
| Max dose for elderly | 2.0 | application/day | 2 | application/day | skin/dermal | Elidel | pimecrolimus | PDR |