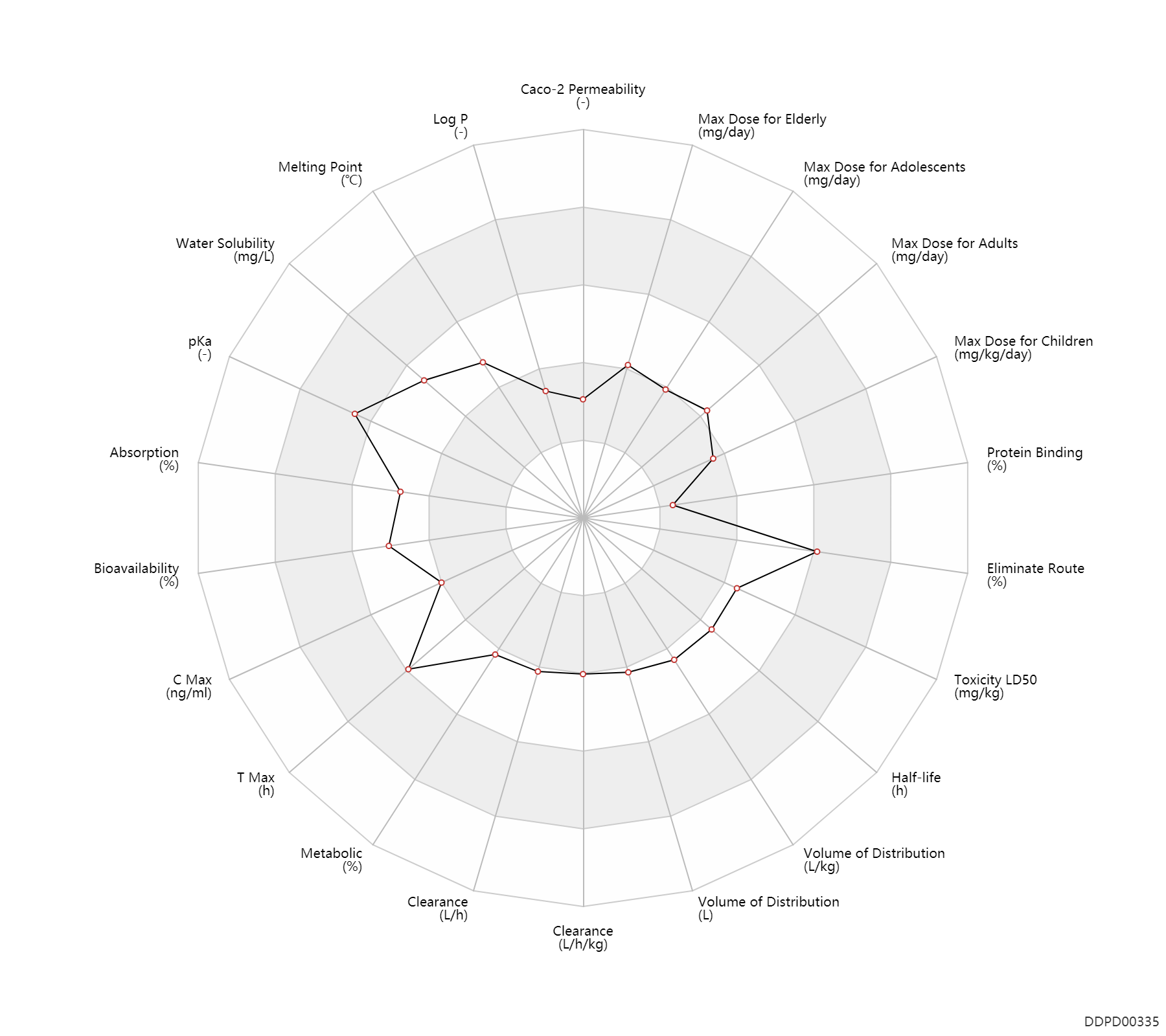
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Reference |
| Caco-2 Permeability |
-6.44 |
- |
-6.44 |
- |
ADME Research, USCD |
| Log P |
0.16 |
- |
0.16 |
- |
HANSCH,C ET AL. (1995) |
| Melting Point |
159.0 |
℃ |
158-160 |
℃ |
Barrett, A.M., Carter, J., Hull, R., Le Count, D.J. and Squire, C.J.; U.S. Patent 3,663,607;
May 16, 1972; assigned to Imperial Chemical Industries Limited, England.
Barrett, A.M., Carter, J., Hull, R., Le Count, D.J. and Squire, C.J.; U.S. Patent 3,836,671;
September 17, 1974; assigned to Imperial Chemical Industries Limited, England. |
| Water Solubility |
13300.0 |
mg/L |
13300 |
mg/L |
MCFARLAND,JW ET AL. 2001) |
| pKa |
9.6 |
- |
9.6 |
- |
MERCK INDEX (2001) |
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| Absorption |
50.0 |
% |
50.0 |
% |
PO, oral; |
|
DRUGBANK |
| Bioavailability |
58.0 |
% |
58±16 |
% |
PO, oral; |
|
The Pharmacological Basis of Therapeutics |
| C Max |
280.0 |
ng/ml |
0.28±0.09 |
mcg/ml |
Oral single dose; |
|
The Pharmacological Basis of Therapeutics |
| T Max |
3.3 |
h |
3.3±1.3 |
h |
Oral single dose; |
|
The Pharmacological Basis of Therapeutics |
| Metabolic |
11.5 |
% |
10-13 |
% |
Liver metabolism; |
|
DRUGBANK |
| Clearance |
8.2 |
L/h |
97.3-176.3 |
ml/min |
Total clearance; |
|
DRUGBANK |
Clearance |
7.9 |
L/h |
95-168 |
ml/min |
Renal clearance; |
|
DRUGBANK |
Clearance |
0.14 |
L/h/kg |
2.4±0.3 |
ml/min/kg |
|
Elderly ↓ ; |
The Pharmacological Basis of Therapeutics |
Clearance |
0.15 |
L/h/kg |
2.5 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
88.2 |
L |
63.8-112.5 |
L |
Total volume of distribution; |
|
DRUGBANK |
Volume of Distribution |
1.3 |
L/kg |
1.3±0.5 |
L/kg |
|
|
The Pharmacological Basis of Therapeutics |
Volume of Distribution |
0.95 |
L/kg |
0.95 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
6.5 |
h |
6-7 |
h |
|
|
DRUGBANK |
Half-life |
6.1 |
h |
6.1±2.0 |
h |
|
RD, renal impairment, Renal disease,including uremia ↑ ;Age ↑ ; |
The Pharmacological Basis of Therapeutics |
Half-life |
6.1 |
h |
6.1 |
h |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 |
2000.0 |
mg/kg |
2.0 |
g/kg |
PO, oral; mouse; |
|
DRUGBANK |
Toxicity LD50 |
57.0 |
mg/kg |
57.0 |
mg/kg |
intravenous injection, IV; mouse; |
|
DRUGBANK |
Toxicity LD50 |
134.0 |
mg/kg |
134.0 |
mg/kg |
Intraperitoneal, IP; mouse; |
|
DRUGBANK |
Toxicity LD50 |
400.0 |
mg/kg |
400.0 |
mg/kg |
subcutaneous injection, SC; mouse; |
|
DRUGBANK |
Toxicity LD50 |
2000.0 |
mg/kg |
2.0 |
g/kg |
PO, oral; Rattus, Rat; |
|
DRUGBANK |
Toxicity LD50 |
77.0 |
mg/kg |
77.0 |
mg/kg |
intravenous injection, IV; Rattus, Rat; |
|
DRUGBANK |
Toxicity LD50 |
600.0 |
mg/kg |
600.0 |
mg/kg |
subcutaneous injection, SC; Rattus, Rat; |
|
DRUGBANK |
Toxicity LD50 |
50.0 |
mg/kg |
50.0 |
mg/kg |
intravenous injection, IV; rabbit; |
|
DRUGBANK |
Toxicity LD50 |
2500.0 |
mg/kg |
2000-3000 |
mg/kg |
PO, oral; mouse; |
|
T3DB |
| Eliminate Route |
85.0 |
% |
85 |
% |
Urinary excretion; intravenous injection, IV; |
|
DRUGBANK |
Eliminate Route |
10.0 |
% |
10 |
% |
Faeces excretion; intravenous injection, IV; |
|
DRUGBANK |
Eliminate Route |
94.0 |
% |
94±8 |
% |
Urinary excretion; Raceme D/L; Unchanged drug; |
|
The Pharmacological Basis of Therapeutics |
| Protein Binding |
11.0 |
% |
6-16 |
% |
|
|
DRUGBANK |
Protein Binding |
5.0 |
% |
<5 |
% |
|
|
The Pharmacological Basis of Therapeutics |