Basic Information
| Drug ID | DDPD00334 |

|
| Drug Name | Olanzapine | |
| Molecular Weight | 312.432 | |
| Molecular Formula | C17H20N4S | |
| CAS Number | 132539-06-1 | |
| SMILES | CN1CCN(CC1)C1=NC2=CC=CC=C2NC2=C1C=C(C)S2 | |
| External Links | ||
| DRUGBANK | DB00334 | |
| T3DB | T3D2754 | |
| PubChem Compound | 4585 | |
| PDR | 2269 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
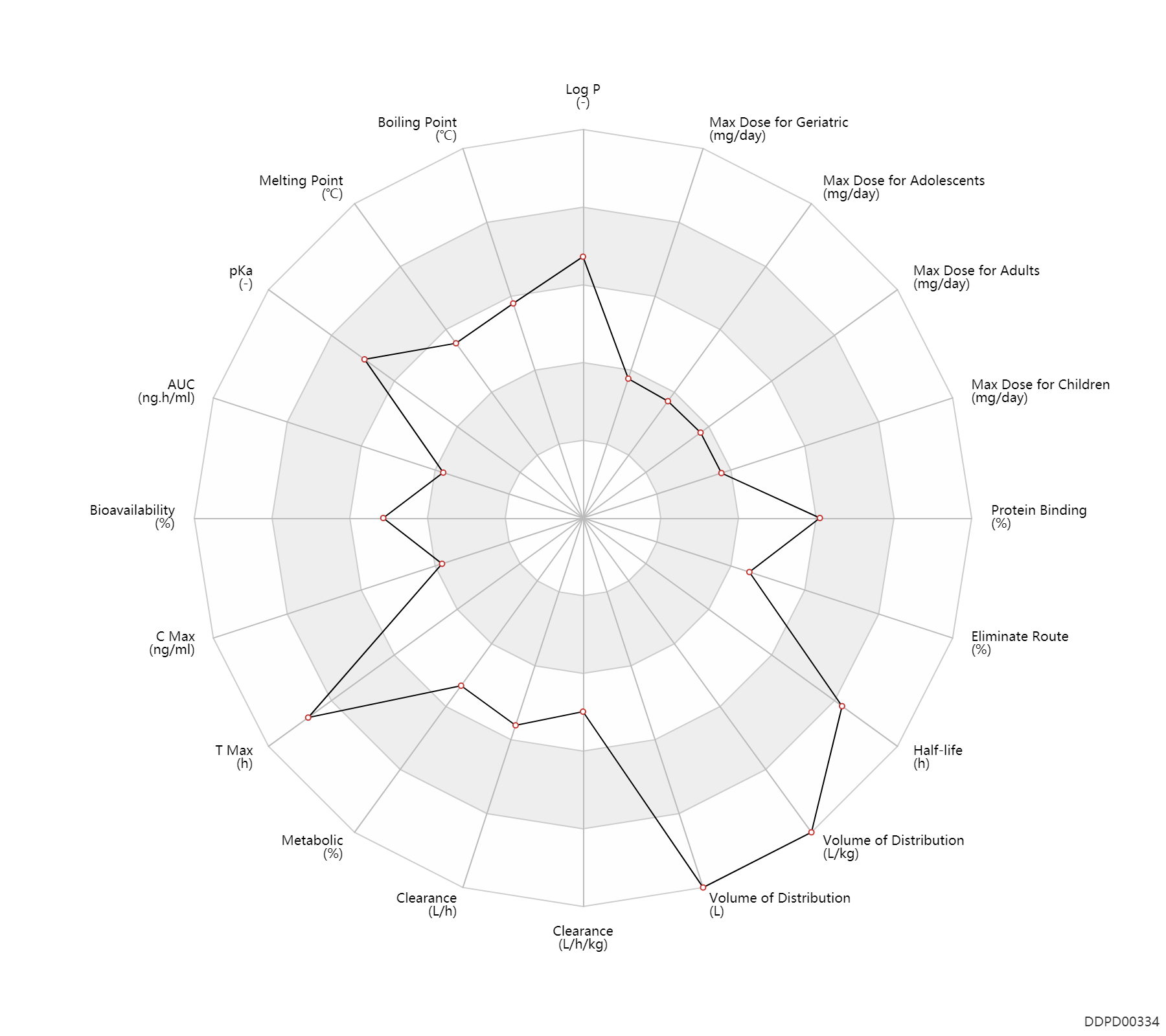
| Log P | 4.094 | - | 4.094 | - | 'MSDS' |
| Boiling Point | 476.0 | ℃ | 476 | ℃ | 'MSDS' |
| Melting Point | 192.0 | ℃ | 189-195 | ℃ | 'MSDS' |
| pKa | 10.57 | - | 10.57 | - | Mylan-Olanzapine product monograph |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| AUC | 333.0 | ng.h/ml | 333.0 | ng.h/ml | DRUGBANK | ||
| Bioavailability | 60.0 | % | ~60 | % | PO, oral; | The Pharmacological Basis of Therapeutics | |
| C Max | 156.9 | ng/ml | 156.9 | ng/ml | PO, oral; food; | food → ; | DRUGBANK | C Max | 12.9 | ng/ml | 12.9±7.5 | ng/ml | Oral single dose; Male, men; normal,healthy; | The Pharmacological Basis of Therapeutics |
| Css | 150.0 | ng/ml | <150 | ng/ml | DRUGBANK | ||
| T Max | 6.0 | h | 6 | h | PO, oral; food; | food → ; | DRUGBANK | T Max | 6.1 | h | 6.1±1.9 | h | Oral single dose; Male, men; normal,healthy; | The Pharmacological Basis of Therapeutics |
| Metabolic | 40.0 | % | 40 | % | Liver metabolism; Inactive metabolite; | DRUGBANK | |
| Clearance | 27.2 | L/h | 25-29.4 | L/h | DRUGBANK | Clearance | 0.37 | L/h/kg | 6.2±2.9 | ml/min/kg | apparent clearance; Male, men; Female, women; schizophrenia; | Hepatic cirrhosis, cirr → ;RD, renal impairment, Renal disease,including uremia → ; | The Pharmacological Basis of Therapeutics |
| Volume of Distribution | 1000.0 | L | 1000.0 | L | DRUGBANK | Volume of Distribution | 16.4 | L/kg | 16.4±5.1 | L/kg | Apparent volume of distribution; Male, men; Female, women; schizophrenia; | The Pharmacological Basis of Therapeutics |
| Half-life | 30.0 | h | 30(21-54) | h | DRUGBANK | Half-life | 33.1 | h | 33.1±10.3 | h | Male, men; Female, women; schizophrenia; patients; | Age ↑ ; | The Pharmacological Basis of Therapeutics |
| Eliminate Route | 53.0 | % | ~53 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 30.0 | % | ~30 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 7.0 | % | 7 | % | Unchanged drug; | DRUGBANK | Eliminate Route | 7.3 | % | 7.3 | % | Urinary excretion; schizophrenia; human, homo sapiens; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 93.0 | % | ~93 | % | plasma proteins; | DRUGBANK | Protein Binding | 93.0 | % | 93 | % | schizophrenia; human, homo sapiens; | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 12.0 | mg/day | 12 | mg/day | PO, oral | Zyprexa | olanzapine | PDR |
| Max dose for children | 20.0 | mg/day | 20 | mg/day | PO, oral | Zyprexa | olanzapine | PDR |
| Max dose for children | 1.0 | mg/day | 20 | mg/day | PO, oral | Zyprexa | olanzapine | PDR |
| Max dose for children | 20.0 | mg/day | 20 | mg/day | PO, oral;IM,intramuscular injection; | Zyprexa | olanzapine | PDR |
| Max dose for children | 20.0 | mg/day | 20 | mg/day | PO, oral;IM,intramuscular injection; | Zyprexa | olanzapine | PDR |
| Max dose for adolescents | 12.0 | mg/day | 12 | mg/day | PO, oral | Zyprexa | olanzapine | PDR |
| Max dose for adolescents | 20.0 | mg/day | 20 | mg/day | PO, oral | Zyprexa | olanzapine | PDR |
| Max dose for adolescents | 20.0 | mg/day | 20 | mg/day | PO, oral;IM,intramuscular injection; | Zyprexa | olanzapine | PDR |
| Max dose for adults | 20.0 | mg/day | 20 | mg/day | PO, oral | Zyprexa | olanzapine | PDR |
| Max dose for adults | 30.0 | mg/day | 30 | mg/day | IM,intramuscular injection | Zyprexa | olanzapine | PDR |
| Max dose for adults | 21.4285714285714 | mg/day | 300 | mg/dose | IM,intramuscular injection | Zyprexa | olanzapine | PDR |
| Max dose for adults | 14.4642857142857 | mg/day | 405 | mg/dose | IM,intramuscular injection | Zyprexa | olanzapine | PDR |
| Max dose for geriatric | 20.0 | mg/day | 20 | mg/day | PO, oral | Zyprexa | olanzapine | PDR |
| Max dose for geriatric | 30.0 | mg/day | 30 | mg/day | IM,intramuscular injection | Zyprexa | olanzapine | PDR |
| Max dose for geriatric | 21.4285714285714 | mg/dose | 300 | mg/dose | IM,intramuscular injection | Zyprexa | olanzapine | PDR |
| Max dose for geriatric | 14.4642857142857 | mg/dose | 405 | mg/dose | IM,intramuscular injection | Zyprexa | olanzapine | PDR |