Basic Information
| Drug ID | DDPD00330 |

|
| Drug Name | Ethambutol | |
| Molecular Weight | 204.3098 | |
| Molecular Formula | C10H24N2O2 | |
| CAS Number | 74-55-5 | |
| SMILES | CC[C@@H](CO)NCCN[C@@H](CC)CO | |
| External Links | ||
| DRUGBANK | DB00330 | |
| PubChem Compound | 14052 | |
| PDR | 2739 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
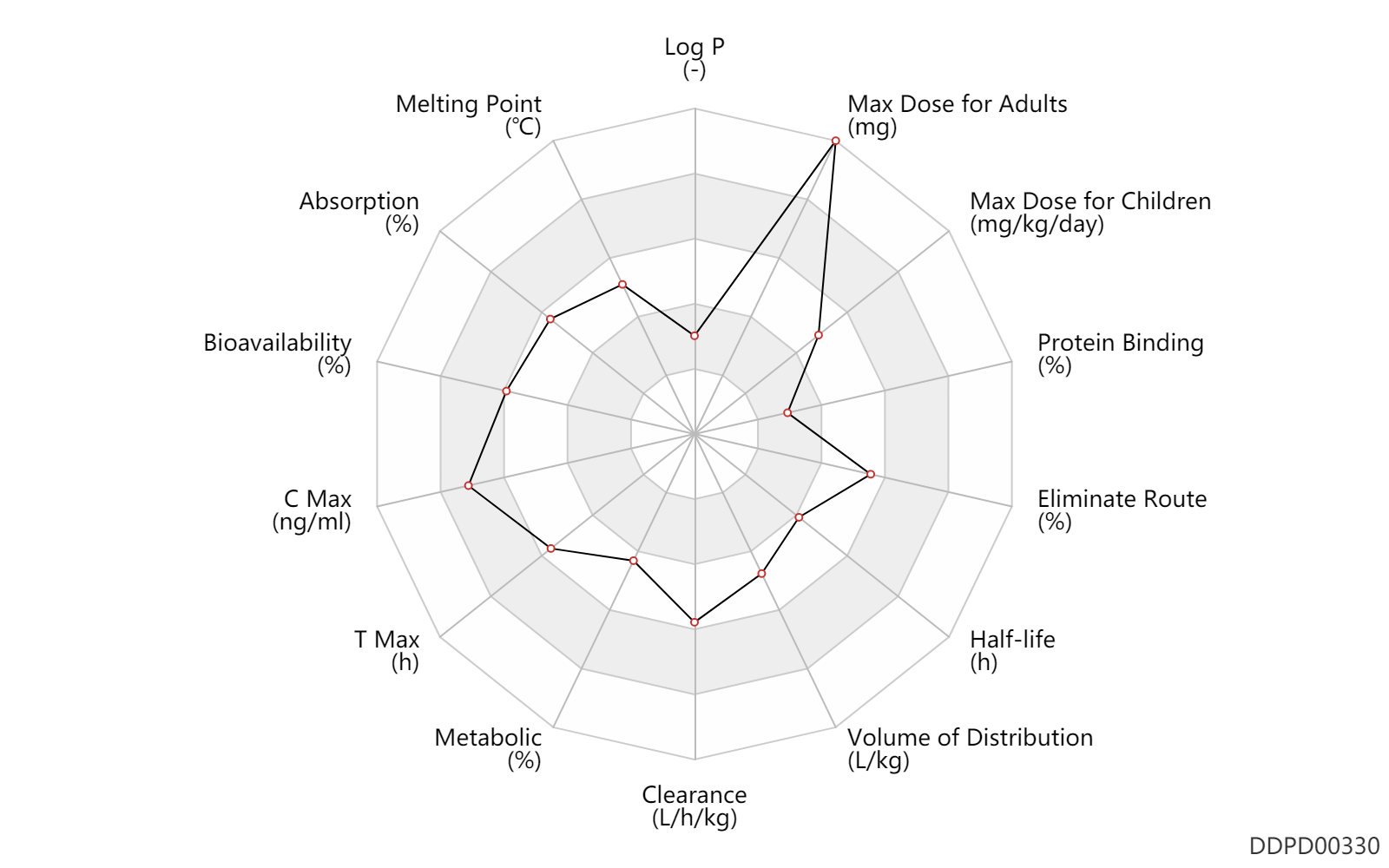
| Log P | -0.3 | - | -0.3 | - | DRUGBANK |
| Melting Point | 173.0 | ℃ | 171.5-174.5 | ℃ | Wilkinson, R.G. and Shepherd, R.G.; U.S. Patent 3,297,707; January 10,1967; assigned to American Cyanamid Company. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Absorption | 77.5 | % | 75-80 | % | PO, oral; | DRUGBANK | |
| Bioavailability | 77.0 | % | 77±8 | % | The Pharmacological Basis of Therapeutics | ||
| C Max | 3500.0 | ng/ml | 2-5 | mcg/ml | The Pharmacological Basis of Therapeutics | ||
| T Max | 3.0 | h | 2-4 | h | Oral single dose; normal,healthy; | The Pharmacological Basis of Therapeutics | |
| Metabolic | 15.0 | % | 15 | % | Liver metabolism; Inactive metabolite; | DRUGBANK | |
| Clearance | 0.52 | L/h/kg | 8.6±0.8 | ml/min/kg | The Pharmacological Basis of Therapeutics | Clearance | 0.60 | L/h/kg | 10 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 1.6 | L/kg | 1.6±0.2 | L/kg | The Pharmacological Basis of Therapeutics | Volume of Distribution | 1.7 | L/kg | 1.7 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 3.5 | h | 3-4 | h | gastroesophageal reflux disease; normal renal function; | DRUGBANK | Half-life | 8.0 | h | 8 | h | gastroesophageal reflux disease; RD, renal impairment, Renal disease,including uremia; | DRUGBANK | Half-life | 3.1 | h | 3.1±0.4 | h | RD, renal impairment, Renal disease,including uremia ↑ ; | The Pharmacological Basis of Therapeutics | Half-life | 3.1 | h | 3.1 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 50.0 | % | ~50 | % | Urinary excretion; PO, oral; Unchanged drug; | DRUGBANK | Eliminate Route | 21.0 | % | 20-22 | % | Faeces excretion; PO, oral; Unchanged drug; | DRUGBANK | Eliminate Route | 79.0 | % | 79±3 | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 25.0 | % | 20-30 | % | DRUGBANK | Protein Binding | 18.0 | % | 6-30 | % | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 25.0 | mg/kg | 25 | mg/kg | PO, oral | qd | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for infants | 2.5 | g | 2.5 | g | PO, oral | qd | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for children | 25.0 | mg/kg/day | 25 | mg/kg/dose | PO, oral | qd | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for children | 2500.0 | mg | 2.5 | g | PO, oral | qd | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for children | 14.2857142857143 | mg/kg/day | 50 | mg/kg/dose | PO, oral | biw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for children | 4000.0 | mg | 4 | g | PO, oral | biw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for adolescents | 25.0 | mg/kg/dose | 25 | mg/kg/dose | PO, oral | qd | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for adolescents | 2500.0 | mg | 2.5 | g | PO, oral | qd | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for adolescents | 50.0 | mg/kg/dose | 50 | mg/kg/dose | PO, oral | biw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for adolescents | 1142.85714285714 | mg | 4 | g | PO, oral | biw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for adolescents | 30.0 | mg/kg/dose | 30 | mg/kg/dose | PO, oral | tiw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for adolescents | 2400.0 | mg | 2.4 | g | PO, oral | tiw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for adults | 25.0 | mg/kg/day | 25 | mg/kg/dose | PO, oral | qd | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for adults | 2500.0 | mg | 2.5 | g | PO, oral | qd | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for adults | 50.0 | mg/kg/dose | 50 | mg/kg/dose | PO, oral | biw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for adults | 4000.0 | mg | 4 | g | PO, oral | biw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for adults | 30.0 | mg/kg/dose | 30 | mg/kg/dose | PO, oral | tiw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for adults | 2400.0 | mg | 2.4 | g | PO, oral | tiw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for elderly | 25.0 | mg/kg/day | 25 | mg/kg/dose | PO, oral | qd | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for elderly | 2500.0 | mg | 2.5 | g | PO, oral | qd | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for elderly | 14.2857142857143 | mg/kg/day | 50 | mg/kg/dose | PO, oral | biw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for elderly | 4000.0 | mg | 4 | g | PO, oral | biw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for elderly | 12.8571428571429 | mg/kg/day | 30 | mg/kg/dose | PO, oral | tiw | Myambutol | ethambutol hydrochloride | PDR |
| Max dose for elderly | 2400.0 | mg | 2.4 | g | PO, oral | tiw | Myambutol | ethambutol hydrochloride | PDR |