Basic Information
| Drug ID | DDPD00315 |

|
| Drug Name | Zolmitriptan | |
| Molecular Weight | 287.3568 | |
| Molecular Formula | C16H21N3O2 | |
| CAS Number | 139264-17-8 | |
| SMILES | CN(C)CCC1=CNC2=CC=C(C[C@H]3COC(=O)N3)C=C12 | |
| External Links | ||
| DRUGBANK | DB00315 | |
| T3DB | T3D2748 | |
| PubChem Compound | 60857 | |
| PDR | 2320 | |
| Drugs.com | Drugs.com Drug Page | |
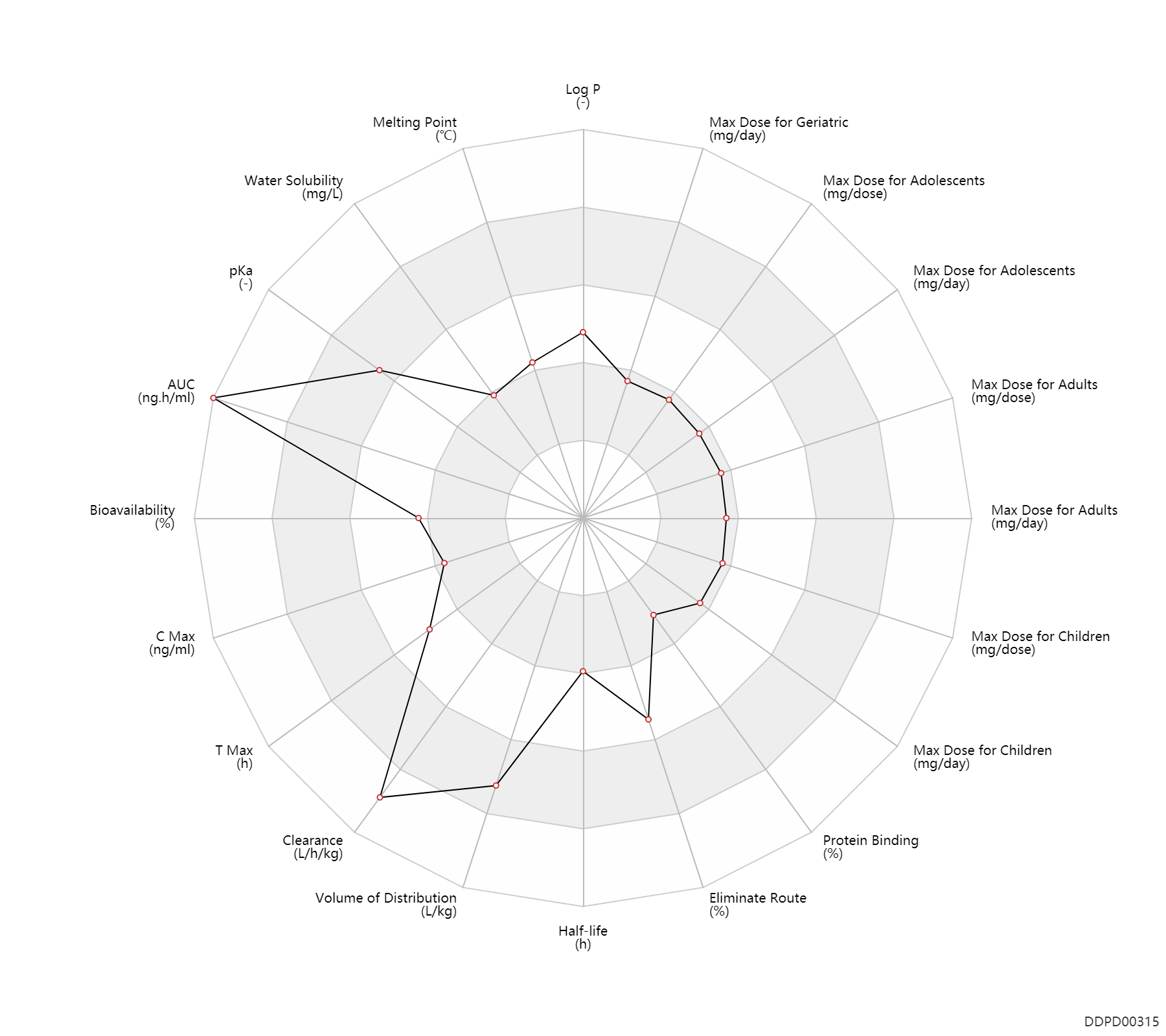
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Log P | 1.792 | - | 1.792 | - | https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=CA&language=en&productNumber=SML0248&brand=SIGMA&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Fsigma%2Fsml0248%3Flang%3Den |
| Melting Point | 136.0 | ℃ | 136 | ℃ | https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/zomig-product-monograph-en.pdf |
| Water Solubility | 1300.0 | mg/L | 1.3 | mg/ml | https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/zomig-product-monograph-en.pdf |
| pKa | 9.64 | - | 9.64 | - | https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/zomig-product-monograph-en.pdf |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| AUC | 129100.0 | ng.h/ml | 84.4-173.8 | ug.h/ml | Tablet, PO, oral; | DRUGBANK | |
| Bioavailability | 40.0 | % | ~40 | % | PO, oral; food; | food → ; | DRUGBANK | Bioavailability | 40.8 | % | 40.8 | % | inhalation, IH; | DRUGBANK |
| C Max | 20.6 | ng/ml | 16-25.2 | ng/ml | Tablet, PO, oral; | DRUGBANK | |
| T Max | 1.5 | h | 1.5 | h | Tablet, PO, oral; | DRUGBANK | T Max | 3.0 | h | 3 | h | Tablet, PO, oral; immediate release formulation; | DRUGBANK |
| Clearance | 1.9 | L/h/kg | 31.5 | ml/min/kg | Tablet, PO, oral; | DRUGBANK | Clearance | 1.6 | L/h/kg | 25.9 | ml/min/kg | inhalation, IH; | DRUGBANK | Clearance | 0.40 | L/h/kg | 6.7 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 7.7 | L/kg | 7-8.4 | L/kg | DRUGBANK | Volume of Distribution | 1.8 | L/kg | 1.8 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 3.0 | h | ~3 | h | elimination half-life; | DRUGBANK | Half-life | 3.5 | h | ~3.5 | h | Active metabolite; | DRUGBANK | Half-life | 3.6 | h | 3.6 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 65.0 | % | ~65 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 30.0 | % | ~30 | % | Faeces excretion; | DRUGBANK |
| Protein Binding | 25.0 | % | ~25 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 5.0 | mg/dose | 5 | mg/dose | intranasal | Zomig Nasal Spray | zolmitriptan | PDR |
| Max dose for children | 10.0 | mg/day | 10 | mg/day | intranasal | Zomig Nasal Spray | zolmitriptan | PDR |
| Max dose for adolescents | 5.0 | mg/dose | 5 | mg/dose | intranasal | Zomig Nasal Spray | zolmitriptan | PDR |
| Max dose for adolescents | 10.0 | mg/day | 10 | mg/day | intranasal | Zomig Nasal Spray | zolmitriptan | PDR |
| Max dose for adults | 5.0 | mg/dose | 5 | mg/dose | PO, oral;intranasal; | Zomig Nasal Spray | zolmitriptan | PDR |
| Max dose for adults | 10.0 | mg/day | 10 | mg/day | PO, oral;intranasal; | Zomig Nasal Spray | zolmitriptan | PDR |
| Max dose for geriatric | 5.0 | mg/dose | 5 | mg/dose | PO, oral;intranasal; | Zomig Nasal Spray | zolmitriptan | PDR |
| Max dose for geriatric | 10.0 | mg/day | 10 | mg/day | PO, oral;intranasal; | Zomig Nasal Spray | zolmitriptan | PDR |