Basic Information
| Drug ID | DDPD00300 |

|
| Drug Name | Tenofovir disoproxil | |
| Molecular Weight | 519.448 | |
| Molecular Formula | C19H30N5O10P | |
| CAS Number | 201341-05-1 | |
| SMILES | [H][C@@](C)(CN1C=NC2=C(N)N=CN=C12)OCP(=O)(OCOC(=O)OC(C)C)OCOC(=O)OC(C)C | |
| External Links | ||
| DRUGBANK | DB00300 | |
| PubChem Compound | 5481350 | |
| PDR | 165 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
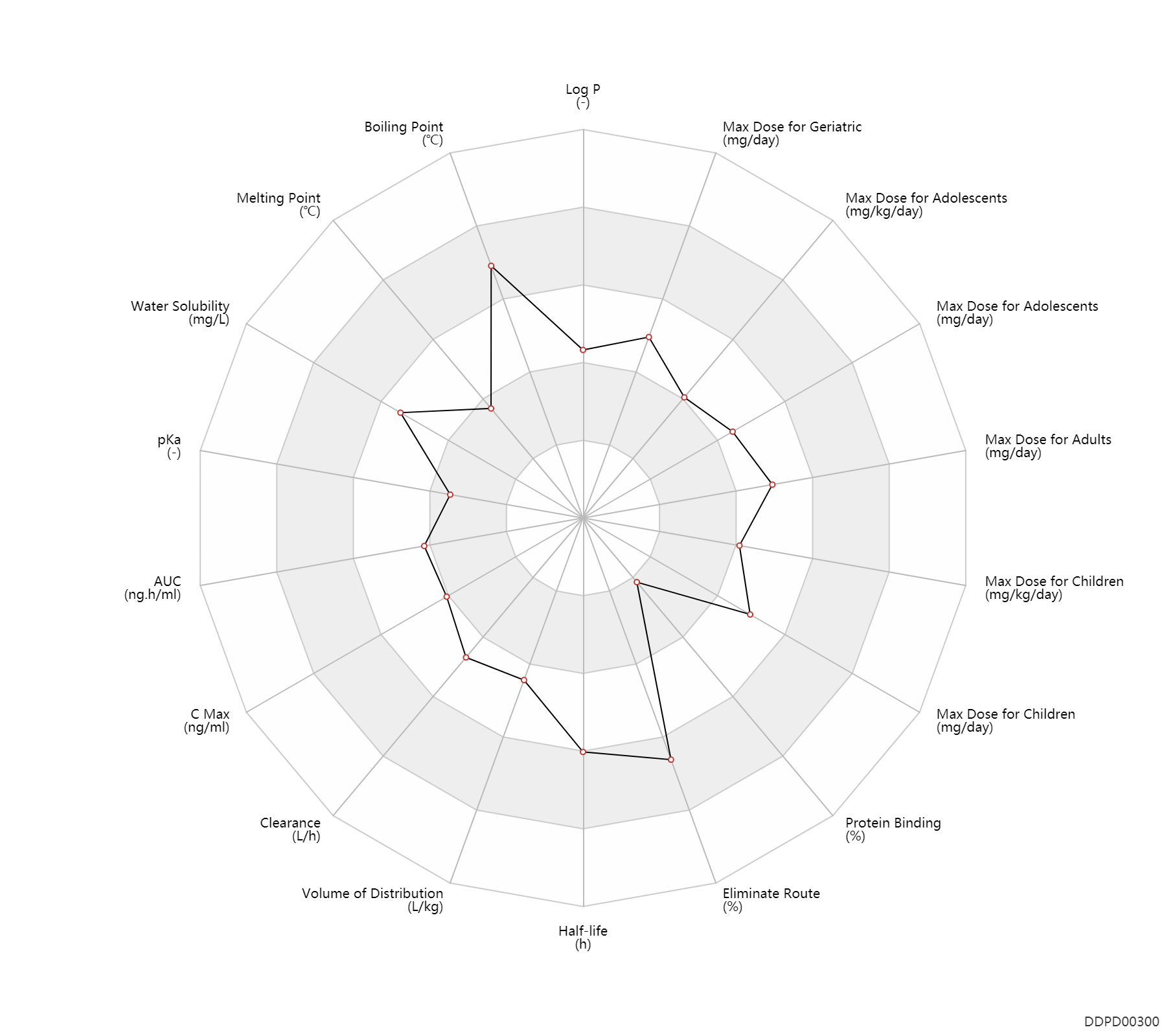
| Log P | 1.25 | - | 1.25 | - | https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021752s005lbl.pdf |
| Boiling Point | 642.7 | ℃ | 642.7 | ℃ | https://www.lookchem.com/Tenofovir-disoproxil-fumarate/ |
| Melting Point | 114.0 | ℃ | 113-115 | ℃ | https://www.chemicalbook.com/ChemicalProductProperty_US_CB7946998.aspx |
| Water Solubility | 13400.0 | mg/L | 13.4 | mg/ml | https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021752s005lbl.pdf |
| pKa | 3.75 | - | 3.75 | - | https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021752s005lbl.pdf |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| AUC | 3320.0 | ng.h/ml | 3.32±1.37 | ug.h/ml | PO, oral; | DRUGBANK |
| C Max | 330.0 | ng/ml | 0.33±0.12 | ug/ml | PO, oral; | DRUGBANK |
| Clearance | 18.0 | L/h | ~300 | ml/min | Total clearance; | DRUGBANK | Clearance | 12.6 | L/h | 210.0 | ml/min | Renal clearance; | DRUGBANK |
| Volume of Distribution | 1.3 | L/kg | 1.3±0.6 | L/kg | at steady state; intravenous injection, IV; | DRUGBANK | Volume of Distribution | 1.2 | L/kg | 1.2±0.4 | L/kg | at steady state; intravenous injection, IV; | DRUGBANK |
| Half-life | 17.0 | h | ~17 | h | terminal half-life; Oral single dose; | DRUGBANK |
| Eliminate Route | 76.0 | % | ~72-80 | % | Urinary excretion; intravenous injection, IV; Unchanged drug; | DRUGBANK |
| Protein Binding | 0.70 | % | <0.7 | % | plasma proteins; high protein binding; human, homo sapiens; | DRUGBANK | Protein Binding | 7.2 | % | <7.2 | % | high protein binding; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 8.0 | mg/kg/day | 8 | mg/kg/day | PO, oral | Viread | tenofovir disoproxil fumarate | PDR |
| Max dose for children | 300.0 | mg/day | 300 | mg/day | PO, oral | Viread | tenofovir disoproxil fumarate | PDR |
| Max dose for children | 8.0 | mg/kg/day | 8 | mg/kg/day | PO, oral | Viread | tenofovir disoproxil fumarate | PDR |
| Max dose for adolescents | 8.0 | mg/kg/day | 8 | mg/kg/day | PO, oral | Viread | tenofovir disoproxil fumarate | PDR |
| Max dose for adolescents | 300.0 | mg/day | 300 | mg/day | PO, oral | Viread | tenofovir disoproxil fumarate | PDR |
| Max dose for adults | 300.0 | mg/day | 300 | mg/day | PO, oral | Viread | tenofovir disoproxil fumarate | PDR |
| Max dose for geriatric | 300.0 | mg/day | 300 | mg/day | PO, oral | Viread | tenofovir disoproxil fumarate | PDR |