Basic Information
| Drug ID | DDPD00283 |

|
| Drug Name | Clemastine | |
| Molecular Weight | 343.89 | |
| Molecular Formula | C21H26ClNO | |
| CAS Number | 15686-51-8 | |
| SMILES | CN1CCC[C@@H]1CCO[C@](C)(C1=CC=CC=C1)C1=CC=C(Cl)C=C1 | |
| External Links | ||
| DRUGBANK | DB00283 | |
| PubChem Compound | 26987 | |
| PDR | 1448 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
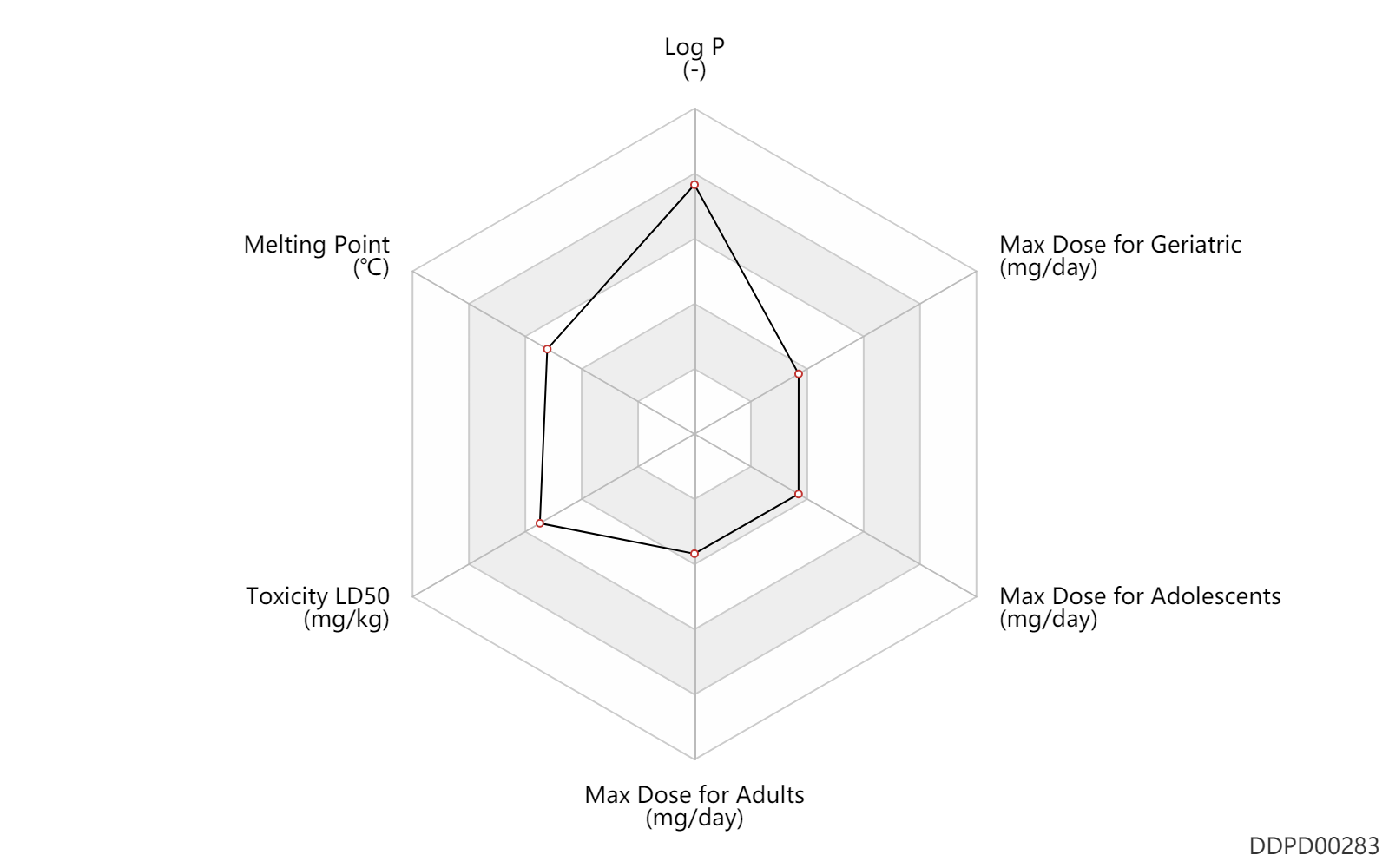
| Log P | 5.2 | - | 5.2 | - | DRUGBANK |
| Melting Point | 178.0 | ℃ | 178 | ℃ | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Toxicity LD50 | 3550.0 | mg/kg | 3550.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 730.0 | mg/kg | 730.0 | mg/kg | PO, oral; mouse; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 0.0 | 0 | Tavist Allergy | clemastine fumarate | PDR | |||
| Max dose for adolescents | 8.04 | mg/day | 8.04 | mg/day | PO, oral | Tavist Allergy | clemastine fumarate | PDR |
| Max dose for adolescents | 6.0 | mg/day | 6 | mg/day | PO, oral | Tavist Allergy | clemastine fumarate | PDR |
| Max dose for adults | 8.04 | mg/day | 8.04 | mg/day | PO, oral | Tavist Allergy | clemastine fumarate | PDR |
| Max dose for adults | 6.0 | mg/day | 6 | mg/day | PO, oral | Tavist Allergy | clemastine fumarate | PDR |
| Max dose for geriatric | 8.04 | mg/day | 8.04 | mg/day | PO, oral | Tavist Allergy | clemastine fumarate | PDR |
| Max dose for geriatric | 6.0 | mg/day | 6 | mg/day | PO, oral | Tavist Allergy | clemastine fumarate | PDR |