Basic Information
| Drug ID | DDPD00279 |

|
| Drug Name | Liothyronine | |
| Molecular Weight | 650.9735 | |
| Molecular Formula | C15H12I3NO4 | |
| CAS Number | 6893-02-3 | |
| SMILES | N[C@@H](CC1=CC(I)=C(OC2=CC(I)=C(O)C=C2)C(I)=C1)C(O)=O | |
| External Links | ||
| DRUGBANK | DB00279 | |
| T3DB | T3D4759 | |
| PubChem Compound | 5920 | |
| PDR | 2347 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
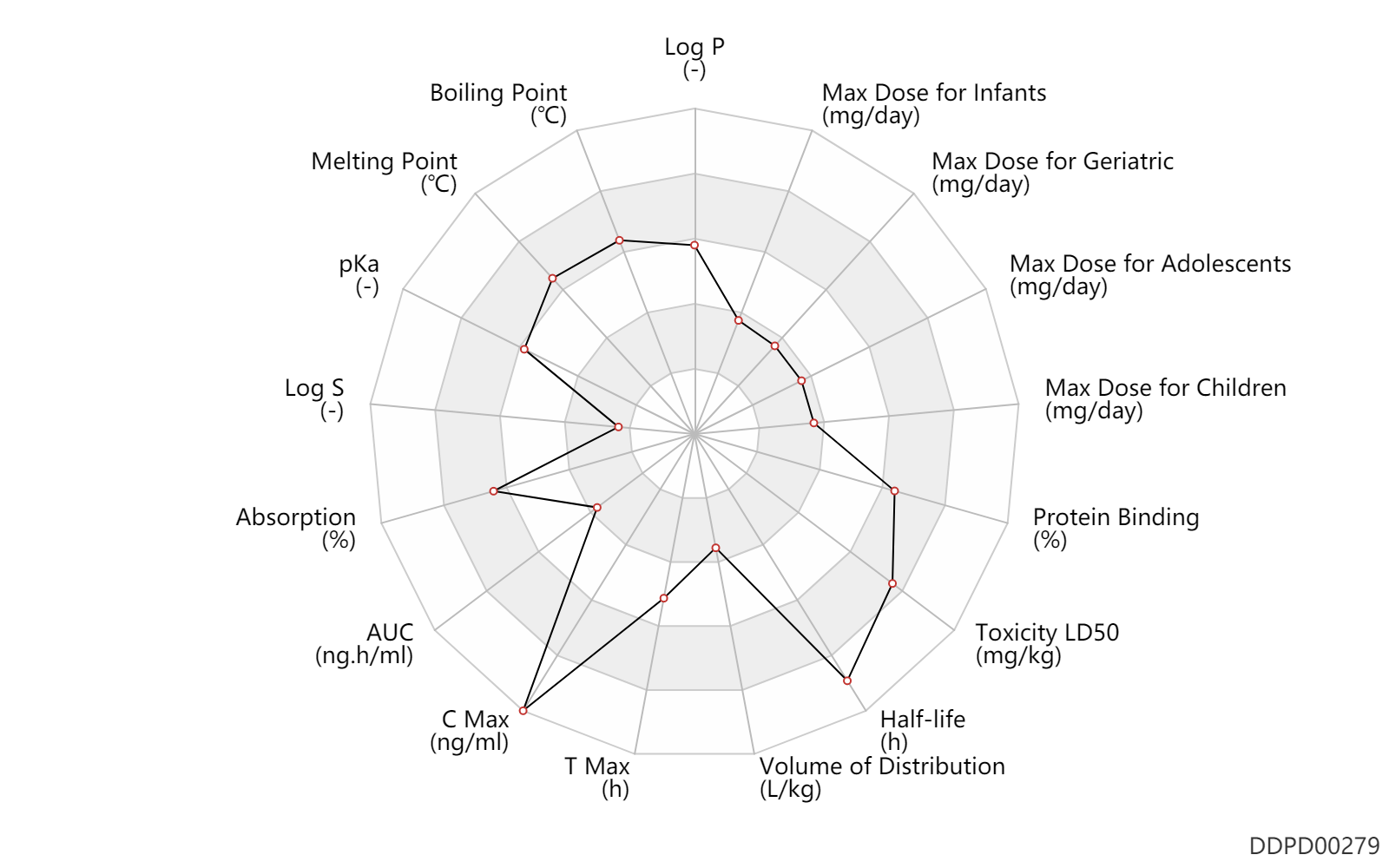
| Log P | 3.0 | - | 3.0 | - | 'MSDS' |
| Boiling Point | 563.0 | ℃ | 563 | ℃ | 'MSDS' |
| Melting Point | 230.0 | ℃ | 230 | ℃ | 'MSDS' |
| pKa | 8.4 | - | 8.4 | - | Harrold M. and Zavod R. 2013. Medicinal Chemistry. |
| Log S | -5.22 | - | -5.22 | - | ADME Research, USCD |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Absorption | 100.0 | % | 100.0 | % | PO, oral; food; | food → ; | DRUGBANK |
| AUC | 47.4 | ng.h/ml | 4742.0 | ng.h/dl | Oral multiple dose; | DRUGBANK | |
| C Max | 34600.0 | ng/ml | 346.0 | ng/dl | Oral multiple dose; | DRUGBANK | |
| T Max | 2.5 | h | 2.5 | h | Oral multiple dose; | DRUGBANK | |
| Volume of Distribution | 0.15 | L/kg | 0.1-0.2 | L/kg | DRUGBANK | ||
| Half-life | 36.0 | h | 1-2 | day | DRUGBANK | ||
| Toxicity LD50 | 4540.0 | mg/kg | >4540 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | |
| Protein Binding | 99.7 | % | ~99.7 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 0.1 | mg/day | 100 | mcg/day | PO, oral | Cytomel | liothyronine sodium | PDR |
| Max dose for children | 0.1 | mg/day | 100 | mcg/day | PO, oral;intravenous injection, IV; | Cytomel | liothyronine sodium | PDR |
| Max dose for adolescents | 0.1 | mg/day | 100 | mcg/day | PO, oral;intravenous injection, IV; | Cytomel | liothyronine sodium | PDR |
| Max dose for geriatric | 0.1 | mg/day | 100 | mcg/day | PO, oral;intravenous injection, IV; | Cytomel | liothyronine sodium | PDR |