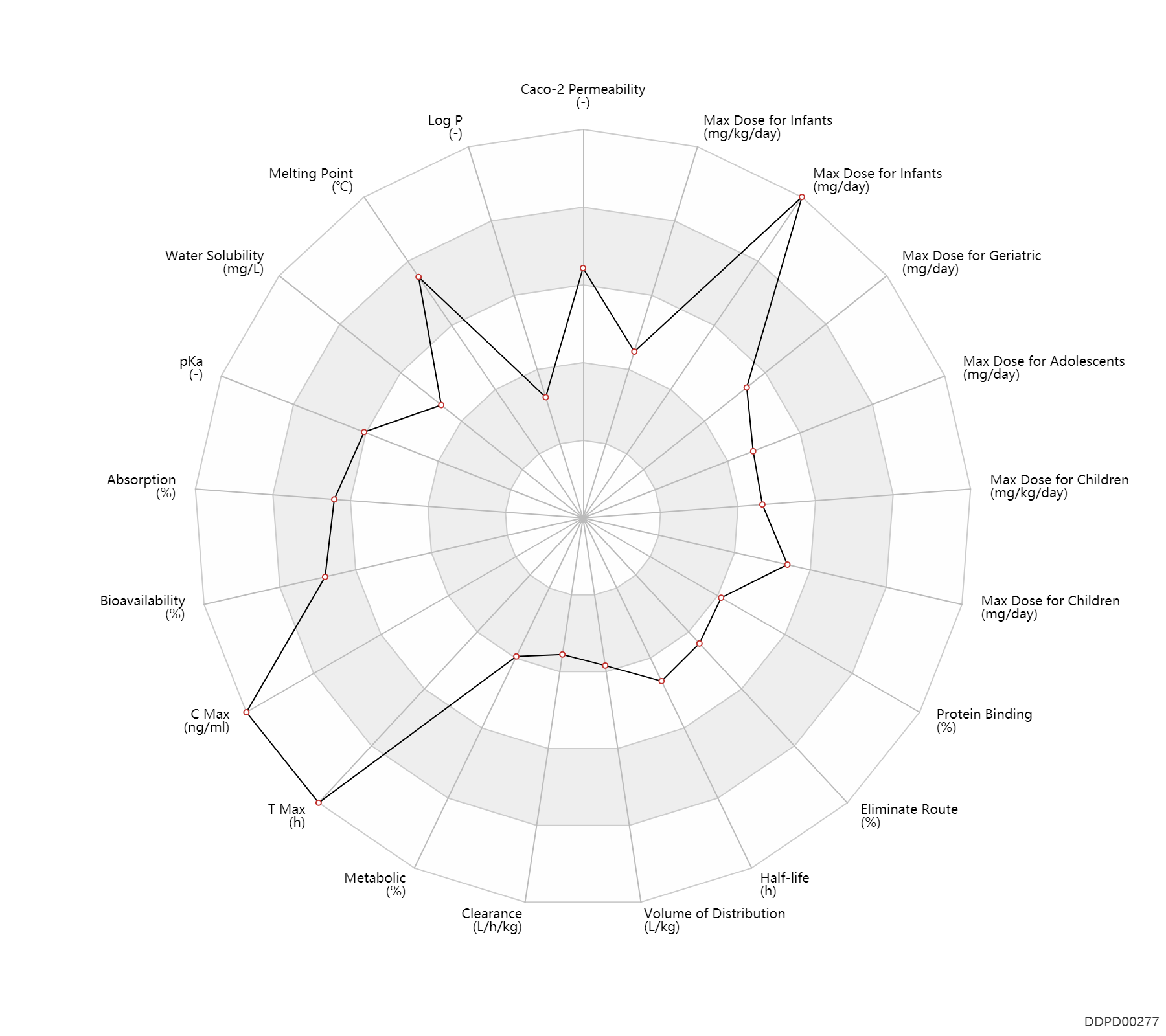
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| Absorption |
100.0 |
% |
100.0 |
% |
PO, oral; |
|
DRUGBANK |
| Bioavailability |
96.0 |
% |
96±8 |
% |
PO, oral; |
|
The Pharmacological Basis of Therapeutics |
| C Max |
7900.0 |
ng/ml |
7.9±0.6 |
mcg/ml |
PO, oral; Drug form; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
C Max |
15000.0 |
ng/ml |
15±2.8 |
mcg/ml |
PO, oral; Drug form; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
C Max |
14000.0 |
ng/ml |
14±3.7 |
mcg/ml |
PO, oral; Drug form; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
| T Max |
1.5 |
h |
~1.5 |
h |
PO, oral; Drug form; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
T Max |
11.5 |
h |
11.5±7.5 |
h |
PO, oral; Drug form; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
T Max |
11.3 |
h |
11.3±4.8 |
h |
PO, oral; Drug form; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
| Metabolic |
6.0 |
% |
6 |
% |
|
|
DRUGBANK |
| Clearance |
0.0279 |
L/h/kg |
0.29-0.64 |
ml/kg/min |
Prem, premature; Neonates; |
|
DRUGBANK |
Clearance |
0.0780 |
L/h/kg |
0.9-1.7 |
ml/kg/min |
Children; |
|
DRUGBANK |
Clearance |
0.0390 |
L/h/kg |
0.65 |
ml/kg/min |
adults; |
|
DRUGBANK |
Clearance |
0.0246 |
L/h/kg |
0.41 |
ml/kg/min |
Elderly; normal,healthy; |
|
DRUGBANK |
Clearance |
0.0198 |
L/h/kg |
0.33 |
ml/kg/min |
lung disease; |
|
DRUGBANK |
Clearance |
0.0324 |
L/h/kg |
0.54 |
ml/kg/min |
Geriatric; COPD, Chronic obstructive pulmonary disease; |
|
DRUGBANK |
Clearance |
0.0288 |
L/h/kg |
0.48 |
ml/kg/min |
COPD, Chronic obstructive pulmonary disease; CP,cor pulmonale; |
|
DRUGBANK |
Clearance |
0.0750 |
L/h/kg |
1.25 |
ml/kg/min |
Cystic fibrosis; |
|
DRUGBANK |
Clearance |
0.0198 |
L/h/kg |
0.31-0.35 |
ml/kg/min |
Hepatitis, Hep; |
|
DRUGBANK |
Clearance |
0.0390 |
L/h/kg |
0.65 |
ml/kg/min |
cholestasis; |
|
DRUGBANK |
Clearance |
0.0282 |
L/h/kg |
0.47 |
ml/kg/min |
Sepsis; multi-organ failure; |
|
DRUGBANK |
Clearance |
0.0228 |
L/h/kg |
0.38 |
ml/kg/min |
LTh hypothyroid; |
|
DRUGBANK |
Clearance |
0.0480 |
L/h/kg |
0.8 |
ml/kg/min |
hyperthyroid, HTh; |
|
DRUGBANK |
Clearance |
0.0390 |
L/h/kg |
0.65±0.20 |
ml/min/kg |
|
Neonates ↓ ;Prem, premature ↓ ;Children ↑ ;Elderly → ;Preg, pregnant → ;Hepatic cirrhosis, cirr ↓ ;CP,cor pulmonale ↓ ;congestive heart disease ↓ ;Hepatitis, Hep ↓ ;LTh hypothyroid ↓ ;Obesity ↓ ;Cystic fibrosis ↑ ;hyperthyroid, HTh ↑ ;RD, renal impairment, Renal disease,including uremia → ;COPD, Chronic obstructive pulmonary disease → ;Somking ↑ ; |
The Pharmacological Basis of Therapeutics |
Clearance |
0.0516 |
L/h/kg |
0.86 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
0.50 |
L/kg |
0.3-0.7 |
L/kg |
|
|
DRUGBANK |
Volume of Distribution |
0.50 |
L/kg |
0.50±0.16 |
L/kg |
|
Elderly → ;Preg, pregnant → ;Prem, premature ↑ ;Obesity ↓ ;Hepatic cirrhosis, cirr → ;RD, renal impairment, Renal disease,including uremia → ;hyperthyroid, HTh → ;LTh hypothyroid → ;Cystic fibrosis ↑ ; |
The Pharmacological Basis of Therapeutics |
Volume of Distribution |
0.51 |
L/kg |
0.51 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
8.0 |
h |
8 |
h |
|
|
DRUGBANK |
Half-life |
9.0 |
h |
9.0±2.1 |
h |
|
Prem, premature ↑ ;Neonates ↑ ;Cystic fibrosis ↓ ;hyperthyroid, HTh ↓ ;Hepatic cirrhosis, cirr ↑ ;congestive heart disease ↑ ;Hepatitis, Hep ↑ ;CP,cor pulmonale ↑ ;LTh hypothyroid ↑ ;RD, renal impairment, Renal disease,including uremia → ;Somking ↓ ;Age → ;normal BMI ↓ ; |
The Pharmacological Basis of Therapeutics |
Half-life |
7.2 |
h |
7.2 |
h |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route |
50.0 |
% |
~50 |
% |
Urinary excretion; Neonates; Unchanged drug; |
|
DRUGBANK |
Eliminate Route |
10.0 |
% |
~10 |
% |
Urinary excretion; Infants; Unchanged drug; |
|
DRUGBANK |
Eliminate Route |
10.0 |
% |
~10 |
% |
Urinary excretion; adults; Unchanged drug; |
|
DRUGBANK |
Eliminate Route |
18.0 |
% |
18±3 |
% |
Urinary excretion; Unchanged drug; |
Neonates ↑ ;Prem, premature ↑ ;Elderly → ;Cystic fibrosis → ; |
The Pharmacological Basis of Therapeutics |
| Protein Binding |
40.0 |
% |
40 |
% |
|
|
DRUGBANK |
Protein Binding |
56.0 |
% |
56±4 |
% |
|
Elderly ↓ ;Neonates ↓ ;Preg, pregnant ↓ ;Hepatic cirrhosis, cirr ↓ ;Obesity ↓ ;Cystic fibrosis → ; |
The Pharmacological Basis of Therapeutics |