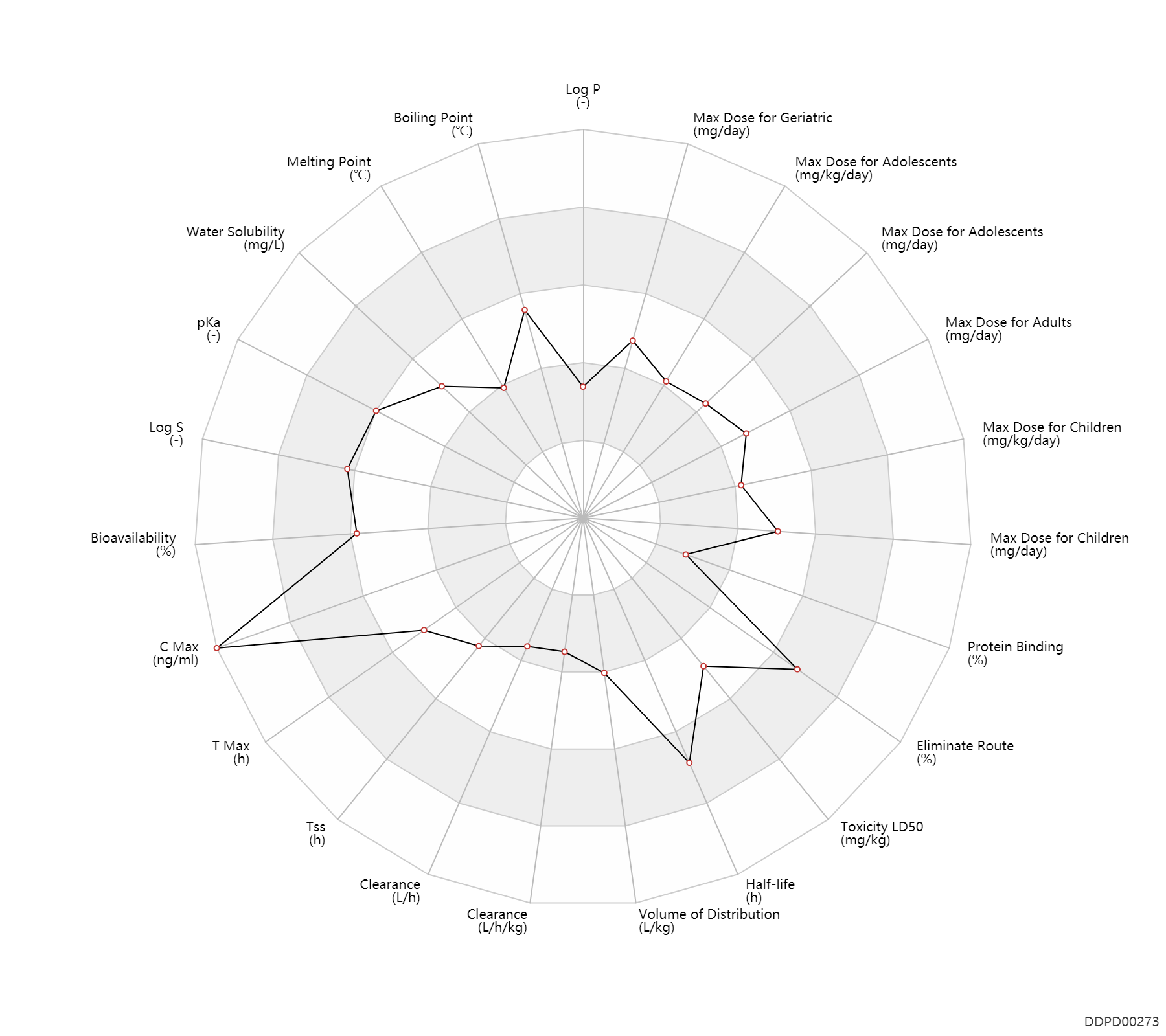
Basic Information
| Drug ID | DDPD00273 |

|
| Drug Name | Topiramate | |
| Molecular Weight | 339.362 | |
| Molecular Formula | C12H21NO8S | |
| CAS Number | 97240-79-4 | |
| SMILES | [H][C@@]12CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@@]3([H])[C@]1([H])OC(C)(C)O2 | |
| External Links | ||
| DRUGBANK | DB00273 | |
| T3DB | T3D2733 | |
| PubChem Compound | 5284627 | |
| PDR | 3313 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Log P | 0.13 | - | 0.13 | - | http://foodb.ca/compounds/FDB023601 |
| Boiling Point | 438.7 | ℃ | 438.7 | ℃ | https://www.lookchem.com/Topiramate/ |
| Melting Point | 124.0 | ℃ | 123-125 | ℃ | https://www.chemicalbook.com/ChemicalProductProperty_US_CB7402630.aspx |
| Water Solubility | 9800.0 | mg/L | 9.8 | mg/ml | https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020505s038s039,020844s032s034lbl.pdf |
| pKa | 8.7 | - | 8.7 | - | http://foodb.ca/compounds/FDB023601 |
| Log S | -1.7 | - | -1.7 | - | http://foodb.ca/compounds/FDB023601 |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 80.0 | % | 80.0 | % | Tablet, PO, oral; | DRUGBANK | Bioavailability | 70.0 | % | ≥70 | % | PO, oral; adults; normal,healthy; epilepsy; | The Pharmacological Basis of Therapeutics |
| C Max | 15215.0 | ng/ml | 1.73-28.7 | ug/ml | PO, oral; | DRUGBANK | C Max | 5500.0 | ng/ml | 5.5±0.6 | mcg/ml | PO, oral; adults; normal,healthy; epilepsy; | The Pharmacological Basis of Therapeutics |
| T Max | 3.1 | h | 1.8-4.3 | h | PO, oral; | DRUGBANK | T Max | 1.7 | h | 1.7±0.6 | h | PO, oral; adults; normal,healthy; epilepsy; | The Pharmacological Basis of Therapeutics |
| Tss | 96.0 | h | 4.0 | day | PO, oral; normal,healthy; | DRUGBANK | |
| Clearance | 1.7 | L/h | 22-36 | ml/min | Plasma clearance; PO, oral; | DRUGBANK | Clearance | 1.1 | L/h | 17-18 | ml/min | Renal clearance; PO, oral; | DRUGBANK | Clearance | 1.5 | L/h | ~20-30 | ml/min | Plasma clearance; PO, oral; adults; | DRUGBANK | Clearance | 0.0246 | L/h/kg | 0.31-0.51 | ml/min/kg | apparent clearance; PO, oral; normal,healthy; Male, men; patients; | Children ↑ ;RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics | Clearance | 0.0162 | L/h/kg | 0.27 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 0.70 | L/kg | 0.6-0.8 | L/kg | Apparent volume of distribution; | DRUGBANK | Volume of Distribution | 0.70 | L/kg | 0.6-0.8 | L/kg | Apparent volume of distribution; PO, oral; normal,healthy; Male, men; patients; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 0.80 | L/kg | 0.8 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 21.0 | h | 19-23 | h | elimination half-life; | DRUGBANK | Half-life | 13.5 | h | 12-15 | h | enzyme-inducers; | DRUGBANK | Half-life | 21.0 | h | 19-23 | h | moderate renal function; severe renal function; | RD, renal impairment, Renal disease,including uremia ↑ ; | The Pharmacological Basis of Therapeutics | Half-life | 34.8 | h | 34.8 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 1500.0 | mg/kg | >1500 | mg/kg | Intraperitoneal, IP; Rattus, Rat; | DRUGBANK | |
| Eliminate Route | 75.0 | % | ~70-80 | % | Urinary excretion; Unchanged drug; | DRUGBANK | Eliminate Route | 83.5 | % | 70-97 | % | Urinary excretion; adults; normal,healthy; epilepsy; human, homo sapiens; human, homo sapiens; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 13.0 | % | 9-17 | % | plasma proteins; | DRUGBANK | Protein Binding | 28.0 | % | 15-41 | % | DRUGBANK | Protein Binding | 15.0 | % | 13-17 | % | adults; normal,healthy; epilepsy; human, homo sapiens; | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 400.0 | mg/day | 400 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for children | 9.0 | mg/kg/day | 9 | mg/kg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for children | 100.0 | mg/day | 100 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for children | 400.0 | mg/day | 400 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for children | 9.0 | mg/kg/day | 9 | mg/kg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for children | 400.0 | mg/day | 400 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for children | 9.0 | mg/kg/day | 9 | mg/kg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for children | 350.0 | mg/day | 350 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for children | 9.0 | mg/kg/day | 9 | mg/kg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for children | 300.0 | mg/day | 300 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for children | 9.0 | mg/kg/day | 9 | mg/kg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for children | 250.0 | mg/day | 250 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for children | 9.0 | mg/kg/day | 9 | mg/kg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for adolescents | 400.0 | mg/day | 400 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for adolescents | 100.0 | mg/day | 100 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for adolescents | 400.0 | mg/day | 400 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for adolescents | 9.0 | mg/kg/day | 9 | mg/kg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for adolescents | 100.0 | mg/day | 100 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for adults | 400.0 | mg/day | 400 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for adults | 100.0 | mg/day | 100 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for geriatric | 400.0 | mg/day | 400 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |
| Max dose for geriatric | 100.0 | mg/day | 100 | mg/day | PO, oral | Trokendi XR | topiramate | PDR |