Basic Information
| Drug ID | DDPD00264 |

|
| Drug Name | Metoprolol | |
| Molecular Weight | 267.3639 | |
| Molecular Formula | C15H25NO3 | |
| CAS Number | 51384-51-1 | |
| SMILES | COCCC1=CC=C(OCC(O)CNC(C)C)C=C1 | |
| External Links | ||
| DRUGBANK | DB00264 | |
| PubChem Compound | 4171 | |
| PDR | 1987 | |
| Drugs.com | Drugs.com Drug Page | |
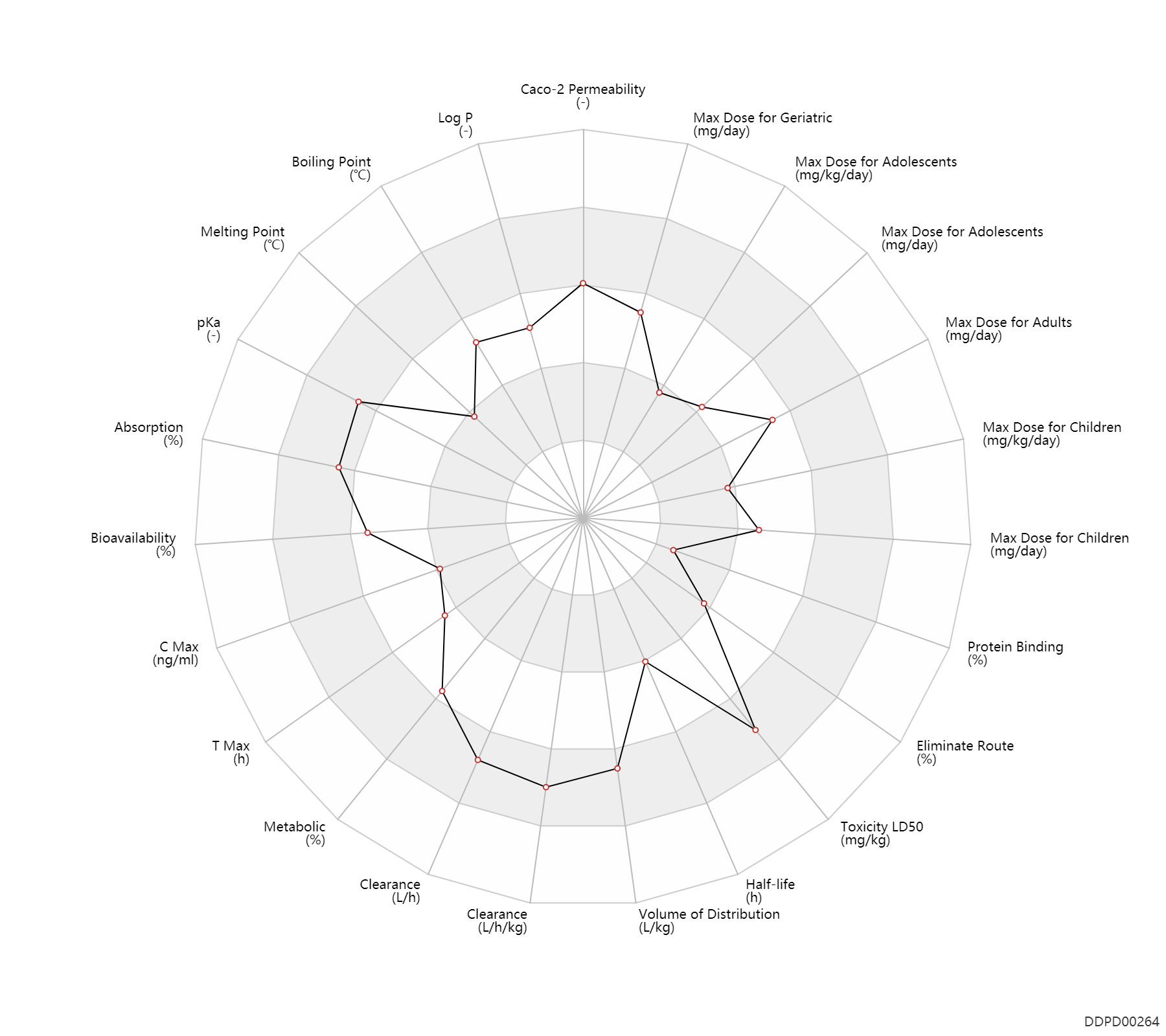
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Caco-2 Permeability | -4.59 | - | -4.59 | - | ADME Research, USCD |
| Log P | 2.15 | - | 2.15 | - | 'MSDS' |
| Boiling Point | 398.0 | ℃ | ~398 | ℃ | 'MSDS' |
| Melting Point | 120.0 | ℃ | 120 | ℃ | 'MSDS' |
| pKa | 9.7 | - | 9.7 | - | Long W. Agilent Technologies. Separation of Beta Blockers at Low and High pH Using Agilent Poroshell HPH C18 |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Absorption | 100.0 | % | ~100 | % | PO, oral; | DRUGBANK | |
| Bioavailability | 100.0 | % | 100.0 | % | intravenous injection, IV; | DRUGBANK | Bioavailability | 38.0 | % | 38±14 | % | PO, oral; | The Pharmacological Basis of Therapeutics |
| C Max | 99.0 | ng/ml | 99±53 | ng/ml | PO, oral; extensive metabolizers, EM; | The Pharmacological Basis of Therapeutics | C Max | 262.0 | ng/ml | 262±29 | ng/ml | PO, oral; poor metabolizers, PM; | The Pharmacological Basis of Therapeutics |
| T Max | 1.5 | h | 1-2 | h | PO, oral; | DRUGBANK | T Max | 0.42 | h | 0.42 | h | intravenous injection, IV; | DRUGBANK | T Max | 2.0 | h | ~2 | h | PO, oral; extensive metabolizers, EM; | The Pharmacological Basis of Therapeutics | T Max | 3.0 | h | ~3 | h | PO, oral; poor metabolizers, PM; | The Pharmacological Basis of Therapeutics |
| Metabolic | 50.0 | % | 50 | % | Liver metabolism; | DRUGBANK | |
| Clearance | 48.0 | L/h | 0.8 | L/min | normal,healthy; patients; | DRUGBANK | Clearance | 36.6 | L/h | 0.61 | L/min | Hepatitis, Hep; patients; | DRUGBANK | Clearance | 0.90 | L/h/kg | 15±3 | ml/min/kg | hydrolysis; | Female, women ↓ ;Elderly → ;Preg, pregnant ↑ ;hyperthyroid, HTh ↑ ;Somking → ; | The Pharmacological Basis of Therapeutics | Clearance | 0.78 | L/h/kg | 13 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 4.2 | L/kg | 4.2 | L/kg | DRUGBANK | Volume of Distribution | 4.2 | L/kg | 4.2±0.7 | L/kg | Female, women ↓ ;Preg, pregnant ↑ ; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 3.1 | L/kg | 3.1 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 5.0 | h | ~3-7 | h | immediate release formulation; | DRUGBANK | Half-life | 3.2 | h | 3.2±0.2 | h | Preg, pregnant → ;Hepatic cirrhosis, cirr ↑ ;Obesity ↑ ;hyperthyroid, HTh → ;Age → ;Somking → ; | The Pharmacological Basis of Therapeutics | Half-life | 3.6 | h | 3.6 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 3880.0 | mg/kg | 3090-4670 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | |
| Eliminate Route | 5.0 | % | <5 | % | Unchanged drug; | DRUGBANK | Eliminate Route | 10.0 | % | 10±3 | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 11.0 | % | ~11 | % | plasma proteins; | DRUGBANK | Protein Binding | 11.0 | % | 11±1 | % | Preg, pregnant → ; | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 2.0 | mg/kg/day | 2 | mg/kg/day | PO, oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for children | 6.0 | mg/kg/day | 6 | mg/kg/day | Tablet,PO,oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for children | 2.4 | mg/kg/day | 2.4 | mg/kg/day | PO, oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for children | 6.0 | mg/kg/day | 6 | mg/kg/day | Tablet,PO,oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for children | 200.0 | mg/day | 200 | mg/day | Tablet,PO,oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for children | 2.4 | mg/kg/day | 2.4 | mg/kg/day | PO, oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for children | 200.0 | mg/day | 200 | mg/day | PO, oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for children | 200.0 | mg/day | 200 | mg/day | Tablet,PO,oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for adolescents | 2.0 | mg/kg/day | 2 | mg/kg/day | PO, oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for adolescents | 6.0 | mg/kg/day | 6 | mg/kg/day | Tablet,PO,oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for adolescents | 2.4 | mg/kg/day | 2.4 | mg/kg/day | PO, oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for adolescents | 200.0 | mg/day | 200 | mg/day | PO, oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for adolescents | 200.0 | mg/day | 200 | mg/day | Tablet,PO,oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for adults | 400.0 | mg/day | 400 | mg/day | Tablet,PO,oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for adults | 450.0 | mg/day | 450 | mg/day | Tablet,PO,oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for geriatric | 400.0 | mg/day | 400 | mg/day | Tablet,PO,oral | Lopressor Tablets | metoprolol tartrate | PDR |
| Max dose for geriatric | 450.0 | mg/day | 450 | mg/day | Tablet,PO,oral | Lopressor Tablets | metoprolol tartrate | PDR |