Basic Information
| Drug ID | DDPD00254 |

|
| Drug Name | Doxycycline | |
| Molecular Weight | 444.4346 | |
| Molecular Formula | C22H24N2O8 | |
| CAS Number | 564-25-0 | |
| SMILES | [H][C@@]12[C@@H](C)C3=CC=CC(O)=C3C(=O)C1=C(O)[C@]1(O)C(=O)C(C(N)=O)=C(O)[C@@H](N(C)C)[C@]1([H])[C@H]2O | |
| External Links | ||
| DRUGBANK | DB00254 | |
| T3DB | T3D3950 | |
| PubChem Compound | 54671203 | |
| PDR | 1579 | |
| Drugs.com | Drugs.com Drug Page | |
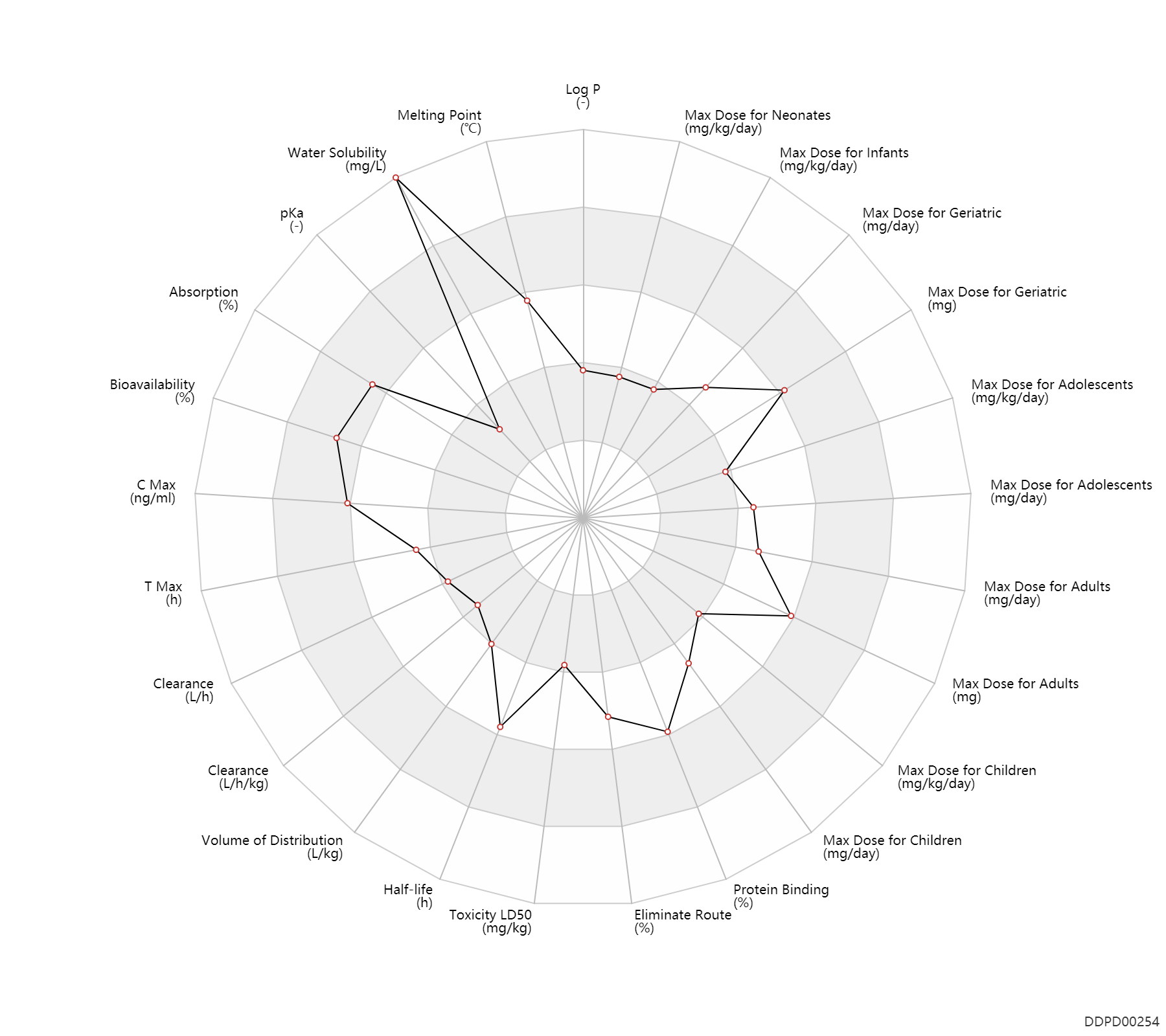
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Log P | 0.63 | - | 0.63 | - | https://deepblue.lib.umich.edu/bitstream/handle/2027.42/64911/21954_ftp.pdf?sequence=1 |
| Melting Point | 201.0 | ℃ | 201 | ℃ | https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/d9891pis.pdf |
| Water Solubility | 50000.0 | mg/L | 50 | mg/ml | https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/d9891pis.pdf |
| pKa | 3.09 | - | 3.09 | - | https://www.ncbi.nlm.nih.gov/pubmed/34018 |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Absorption | 100.0 | % | ~100 | % | PO, oral; food; | food → ; | DRUGBANK |
| Bioavailability | 93.0 | % | 93.0 | % | PO, oral; | The Pharmacological Basis of Therapeutics | |
| C Max | 2600.0 | ng/ml | 2.6 | mcg/ml | Oral single dose; | DRUGBANK | C Max | 1850.0 | ng/ml | 1.7-2 | mcg/ml | PO, oral; adults; | The Pharmacological Basis of Therapeutics | C Max | 2800.0 | ng/ml | 2.8 | mcg/ml | adults; | The Pharmacological Basis of Therapeutics |
| T Max | 2.0 | h | 2 | h | Oral single dose; | DRUGBANK | T Max | 1.5 | h | 1-2 | h | PO, oral; adults; | The Pharmacological Basis of Therapeutics |
| Clearance | 4.5 | L/h | ~75 | ml/min | normal,healthy; | DRUGBANK | Clearance | 0.0318 | L/h/kg | 0.53±0.18 | ml/min/kg | Elderly ↓ ;RD, renal impairment, Renal disease,including uremia → ;HL,hyperlipoproteinemia ↓ ; | The Pharmacological Basis of Therapeutics | Clearance | 0.0276 | L/h/kg | 0.46 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 0.70 | L/kg | 0.7 | L/kg | DRUGBANK | Volume of Distribution | 0.75 | L/kg | 0.75±0.32 | L/kg | Elderly ↓ ;HL,hyperlipoproteinemia ↓ ; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 0.69 | L/kg | 0.69 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 16.3 | h | 16.33±4.53 | h | DRUGBANK | Half-life | 16.0 | h | 16±6 | h | RD, renal impairment, Renal disease,including uremia → ;HL,hyperlipoproteinemia → ;Age → ; | The Pharmacological Basis of Therapeutics | Half-life | 14.0 | h | 14 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 262.0 | mg/kg | 262.0 | mg/kg | Intraperitoneal, IP; Rattus, Rat; | T3DB | |
| Eliminate Route | 50.0 | % | 40-60 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 30.0 | % | 30 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 41.0 | % | 41±19 | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 90.0 | % | >90 | % | DRUGBANK | Protein Binding | 88.0 | % | 88±5 | % | RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 4.4 | mg/kg/day | 4.4 | mg/kg/day | PO, oral;intravenous injection, IV; | Oracea | doxycycline | PDR |
| Max dose for infants | 4.4 | mg/kg/day | 4.4 | mg/kg/day | PO, oral;intravenous injection, IV; | Oracea | doxycycline | PDR |
| Max dose for children | 300.0 | mg/day | 300 | mg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for children | 600.0 | mg | 600 | mg | PO, oral | Oracea | doxycycline | PDR |
| Max dose for children | 300.0 | mg/day | 300 | mg/day | intravenous injection, IV | Oracea | doxycycline | PDR |
| Max dose for children | 240.0 | mg/day | 240 | mg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for children | 720.0 | mg | 720 | mg | PO, oral | Oracea | doxycycline | PDR |
| Max dose for children | 40.0 | mg/day | 40 | mg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for children | 4.4 | mg/kg/day | 4.4 | mg/kg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for children | 4.4 | mg/kg/day | 4.4 | mg/kg/day | intravenous injection, IV | Oracea | doxycycline | PDR |
| Max dose for children | 5.3 | mg/kg/day | 5.3 | mg/kg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for children | 4.4 | mg/kg/day | 4.4 | mg/kg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for children | 4.4 | mg/kg/day | 4.4 | mg/kg/day | intravenous injection, IV | Oracea | doxycycline | PDR |
| Max dose for children | 5.3 | mg/kg/day | 5.3 | mg/kg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for adolescents | 300.0 | mg/day | 300 | mg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for adolescents | 600.0 | mg | 600 | mg | PO, oral | Oracea | doxycycline | PDR |
| Max dose for adolescents | 300.0 | mg/day | 300 | mg/day | intravenous injection, IV | Oracea | doxycycline | PDR |
| Max dose for adolescents | 240.0 | mg/day | 240 | mg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for adolescents | 720.0 | mg | 720 | mg | PO, oral | Oracea | doxycycline | PDR |
| Max dose for adolescents | 4.4 | mg/kg/day | 4.4 | mg/kg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for adolescents | 4.4 | mg/kg/day | 4.4 | mg/kg/day | intravenous injection, IV | Oracea | doxycycline | PDR |
| Max dose for adolescents | 5.3 | mg/kg/day | 5.3 | mg/kg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for adults | 300.0 | mg/day | 300 | mg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for adults | 600.0 | mg | 600 | mg | PO, oral | Oracea | doxycycline | PDR |
| Max dose for adults | 300.0 | mg/day | 300 | mg/day | intravenous injection, IV | Oracea | doxycycline | PDR |
| Max dose for adults | 240.0 | mg/day | 240 | mg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for adults | 720.0 | mg | 720 | mg | PO, oral | Oracea | doxycycline | PDR |
| Max dose for adults | 40.0 | mg/day | 40 | mg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for geriatric | 300.0 | mg/day | 300 | mg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for geriatric | 600.0 | mg | 600 | mg | PO, oral | Oracea | doxycycline | PDR |
| Max dose for geriatric | 300.0 | mg/day | 300 | mg/day | intravenous injection, IV | Oracea | doxycycline | PDR |
| Max dose for geriatric | 240.0 | mg/day | 240 | mg/day | PO, oral | Oracea | doxycycline | PDR |
| Max dose for geriatric | 720.0 | mg | 720 | mg | PO, oral | Oracea | doxycycline | PDR |
| Max dose for geriatric | 40.0 | mg/day | 40 | mg/day | PO, oral | Oracea | doxycycline | PDR |