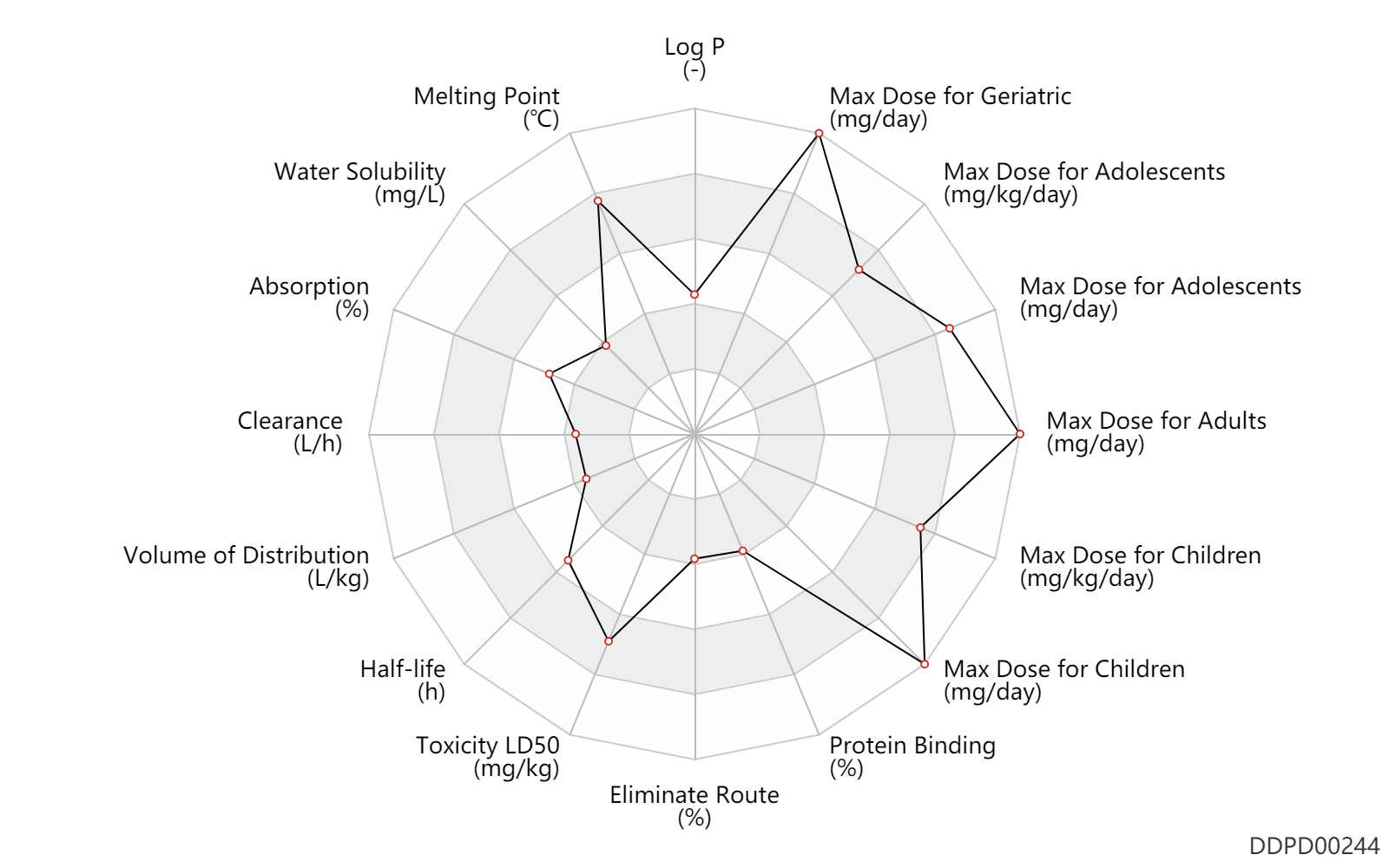
Basic Information
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Log P | 1.2 | - | 1.2 | - | DRUGBANK |
| Melting Point | 283.0 | ℃ | 283 | ℃ | PhysProp |
| Water Solubility | 840.0 | mg/L | 0.84 | g/L | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Absorption | 25.0 | % | 20-30 | % | Capsule, PO, Oral; | DRUGBANK | Absorption | 80.0 | % | ~80 | % | Liquid; | DRUGBANK |
| Clearance | 2.1 | L/h | 2.05 | L/h | Renal clearance; fasting; young; | DRUGBANK | Clearance | 2.1 | L/h | 2.04-2.13 | L/h | Renal clearance; fasting; Elderly; | DRUGBANK |
| Volume of Distribution | 0.20 | L/kg | ~0.2 | L/kg | Steady state volume of distribution; adults; | DRUGBANK |
| Half-life | 9.5 | h | 7-12 | h | elimination half-life; PO, oral; extended release formulation; | DRUGBANK | Half-life | 17.5 | h | 12-23 | h | elimination half-life; PO, oral; extended release formulation; Active metabolite; Metabolite; | DRUGBANK |
| Toxicity LD50 | 3370.0 | mg/kg | 3370.0 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 2800.0 | mg/kg | 2800.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 5000.0 | mg/kg | >5 | g/kg | skin/dermal; rabbit; | DRUGBANK |
| Eliminate Route | 8.0 | % | <8 | % | Urinary excretion; PO, oral; extended release formulation; Unchanged drug; | DRUGBANK |
| Protein Binding | 43.0 | % | ~43 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 44.0 | mg/kg/day | 44 | mg/kg/day | PO, oral | Canasa | mesalamine | PDR |
| Max dose for children | 2400.0 | mg/day | 2.4 | g/day | PO, oral | Canasa | mesalamine | PDR |
| Max dose for children | 61.0 | mg/kg/day | 61 | mg/kg/day | PO, oral | Canasa | mesalamine | PDR |
| Max dose for children | 2000.0 | mg/day | 2 | g/day | PO, oral | Canasa | mesalamine | PDR |
| Max dose for children | 71.0 | mg/kg/day | 71 | mg/kg/day | PO, oral | Canasa | mesalamine | PDR |
| Max dose for children | 1200.0 | mg/day | 1.2 | g/day | PO, oral | Canasa | mesalamine | PDR |
| Max dose for adolescents | 44.0 | mg/kg/day | 44 | mg/kg/day | PO, oral | Canasa | mesalamine | PDR |
| Max dose for adolescents | 2400.0 | mg/day | 2.4 | g/day | PO, oral | Canasa | mesalamine | PDR |
| Max dose for adolescents | 61.0 | mg/kg/day | 61 | mg/kg/day | PO, oral | Canasa | mesalamine | PDR |
| Max dose for adolescents | 2000.0 | mg/day | 2 | g/day | PO, oral | Canasa | mesalamine | PDR |
| Max dose for adolescents | 71.0 | mg/kg/day | 71 | mg/kg/day | PO, oral | Canasa | mesalamine | PDR |
| Max dose for adolescents | 1200.0 | mg/day | 1.2 | g/day | PO, oral | Canasa | mesalamine | PDR |
| Max dose for adults | 4000.0 | mg/day | 4 | g/day | Rectal Administration | Canasa | mesalamine | PDR |
| Max dose for adults | 1500.0 | mg/day | 1.5 | g/day | Rectal Administration | Canasa | mesalamine | PDR |
| Max dose for adults | 1500.0 | mg/day | 1.5 | g/day | Capsule, PO, Oral | Canasa | mesalamine | PDR |
| Max dose for adults | 2400.0 | mg/day | 2.4 | g/day | Tablet,PO,oral;Capsule, PO, Oral; | Canasa | mesalamine | PDR |
| Max dose for adults | 4000.0 | mg/day | 4 | g/day | Capsule, PO, Oral | Canasa | mesalamine | PDR |
| Max dose for adults | 4800.0 | mg/day | 4.8 | g/day | Tablet,PO,oral | Canasa | mesalamine | PDR |
| Max dose for geriatric | 4000.0 | mg/day | 4 | g/day | Rectal Administration | Canasa | mesalamine | PDR |
| Max dose for geriatric | 1500.0 | mg/day | 1.5 | g/day | Rectal Administration | Canasa | mesalamine | PDR |
| Max dose for geriatric | 1500.0 | mg/day | 1.5 | g/day | Capsule, PO, Oral | Canasa | mesalamine | PDR |
| Max dose for geriatric | 2400.0 | mg/day | 2.4 | g/day | Tablet,PO,oral;Capsule, PO, Oral; | Canasa | mesalamine | PDR |
| Max dose for geriatric | 4000.0 | mg/day | 4 | g/day | Capsule, PO, Oral | Canasa | mesalamine | PDR |
| Max dose for geriatric | 4800.0 | mg/day | 4.8 | g/day | Tablet,PO,oral | Canasa | mesalamine | PDR |