Basic Information
| Drug ID | DDPD00213 |

|
| Drug Name | Pantoprazole | |
| Molecular Weight | 383.37 | |
| Molecular Formula | C16H15F2N3O4S | |
| CAS Number | 102625-70-7 | |
| SMILES | COC1=C(OC)C(CS(=O)C2=NC3=C(N2)C=C(OC(F)F)C=C3)=NC=C1 | |
| External Links | ||
| DRUGBANK | DB00213 | |
| PubChem Compound | 4679 | |
| PDR | 2095 | |
| Drugs.com | Drugs.com Drug Page | |
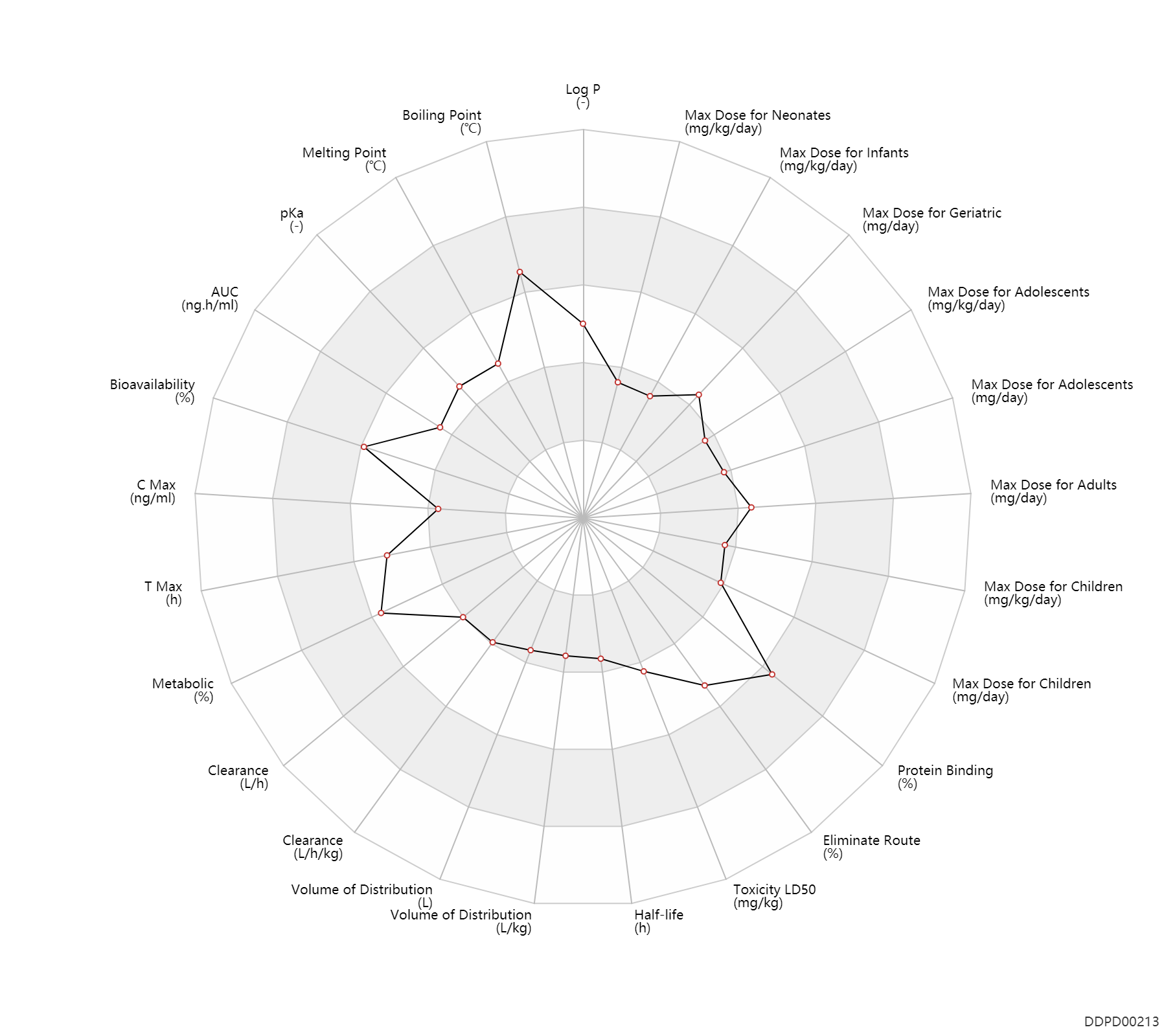
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Log P | 2.05 | - | 2.05 | - | https://www.pfizer.com/sites/default/files/products/material_safety_data/Pantoprazo;le_sodium_for_injection_20-June-2016.pdf |
| Boiling Point | 586.9 | ℃ | 586.9±60.0 | ℃ | http://www.chemspider.com/Chemical-Structure.4517.html |
| Melting Point | 149.5 | ℃ | 149-150 | ℃ | https://www.pfizer.com/sites/default/files/products/material_safety_data/Pantoprazo;le_sodium_for_injection_20-June-2016.pdf |
| pKa | 3.92 | - | 3.92,8.19 | - | https://www.ema.europa.eu/documents/assessment-report/pantozol-control-epar-public-assessment-report_en.pdf | pKa | 8.19 | - | 3.92,8.19 | - | https://www.ema.europa.eu/documents/assessment-report/pantozol-control-epar-public-assessment-report_en.pdf |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| AUC | 5000.0 | ng.h/ml | 5.0 | ug.h/ml | Oral single dose; food; | food → ; | DRUGBANK |
| Bioavailability | 77.0 | % | 77.0 | % | PO, oral; increasing dosing frequency; | increasing dosing frequency → ; | DRUGBANK |
| C Max | 2.5 | ng/ml | ~2.5 | ug/L | Oral single dose; food; | food → ; | DRUGBANK |
| T Max | 2.5 | h | 2-3 | h | PO, oral; increasing dosing frequency; | increasing dosing frequency → ; | DRUGBANK | T Max | 2.5 | h | 2-3 | h | Oral single dose; food; | food → ; | DRUGBANK |
| Metabolic | 80.0 | % | 80 | % | Urinary excretion; PO, oral; intravenous injection, IV; | DRUGBANK | Metabolic | 20.0 | % | 20 | % | Faeces excretion; PO, oral; intravenous injection, IV; | DRUGBANK |
| Clearance | 10.8 | L/h | 7.6-14.0 | L/h | Total clearance; intravenous injection, IV; extensive metabolizers, EM; adults; | gaining weight ↑ ; | DRUGBANK | Clearance | 2.4 | L/h | 2.4 | L/h | gastroesophageal reflux disease; Children; | DRUGBANK | Clearance | 0.13 | L/h/kg | 2.2 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 17.3 | L | 11-23.6 | L | Apparent volume of distribution; | DRUGBANK | Volume of Distribution | 0.17 | L/kg | 0.17 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 1.0 | h | ~1 | h | DRUGBANK | Half-life | 1.9 | h | 1.9 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 747.0 | mg/kg | 747.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | |
| Eliminate Route | 71.0 | % | ~71 | % | Urinary excretion; Oral single dose; intravenous injection, IV; normal,healthy; | DRUGBANK | Eliminate Route | 18.0 | % | 18 | % | Faeces excretion; Oral single dose; intravenous injection, IV; normal,healthy; | DRUGBANK |
| Protein Binding | 98.0 | % | ~98 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 1.2 | mg/kg/day | 1.2 | mg/kg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for infants | 1.2 | mg/kg/day | 1.2 | mg/kg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for children | 40.0 | mg/day | 40 | mg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for children | 80.0 | mg/day | 80 | mg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for children | 20.0 | mg/day | 20 | mg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for children | 2.5 | mg/kg/day | 2.5 | mg/kg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for children | 80.0 | mg/day | 80 | mg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for children | 2.5 | mg/kg/day | 2.5 | mg/kg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for adolescents | 40.0 | mg/day | 40 | mg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for adolescents | 80.0 | mg/day | 80 | mg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for adolescents | 20.0 | mg/day | 20 | mg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for adolescents | 2.5 | mg/kg/day | 2.5 | mg/kg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for adolescents | 80.0 | mg/day | 80 | mg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for adults | 80.0 | mg/day | 80 | mg/day | PO, oral;intravenous injection, IV; | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for adults | 160.0 | mg/day | 160 | mg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for adults | 240.0 | mg/day | 240 | mg/day | PO, oral;intravenous injection, IV; | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for geriatric | 80.0 | mg/day | 80 | mg/day | PO, oral;intravenous injection, IV; | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for geriatric | 160.0 | mg/day | 160 | mg/day | PO, oral | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |
| Max dose for geriatric | 240.0 | mg/day | 240 | mg/day | PO, oral;intravenous injection, IV; | Protonix Delayed Release Oral Suspension and Tablets | pantoprazole sodium | PDR |