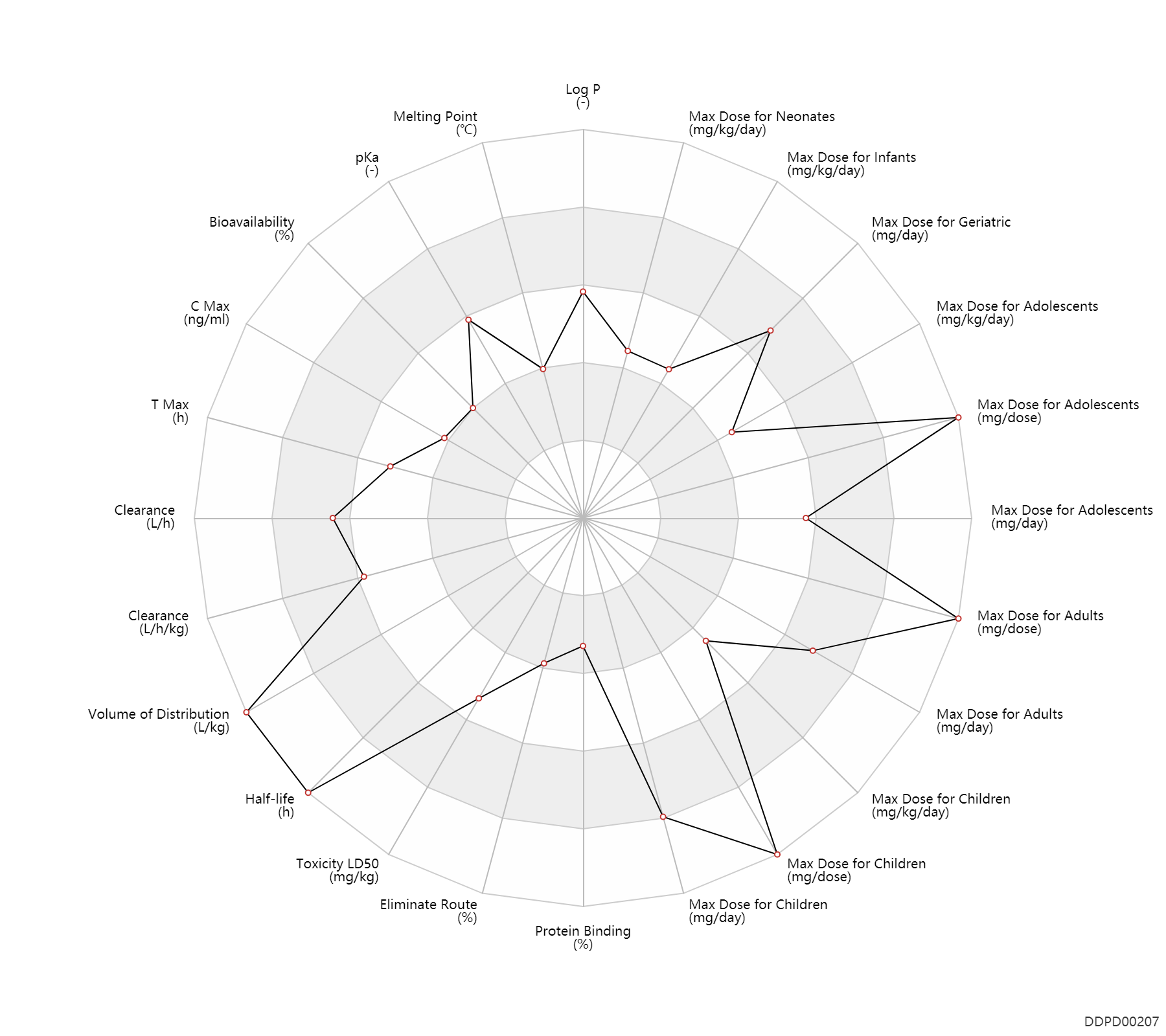
| Max dose for neonates |
20.0 |
mg/kg/day |
20 |
mg/kg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for infants |
10.0 |
mg/kg/day |
10 |
mg/kg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for infants |
30.0 |
mg/kg |
30 |
mg/kg |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for infants |
20.0 |
mg/kg/day |
20 |
mg/kg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for infants |
60.0 |
mg/kg |
60 |
mg/kg |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for infants |
10.0 |
mg/kg/day |
10 |
mg/kg/day |
|
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for infants |
20.0 |
mg/kg/day |
20 |
mg/kg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for infants |
10.0 |
mg/kg/day |
10 |
mg/kg/day |
intravenous injection, IV |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for infants |
20.0 |
mg/kg/day |
20 |
mg/kg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
12.0 |
mg/kg/day |
12 |
mg/kg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
30.0 |
mg/kg |
30 |
mg/kg |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
20.0 |
mg/kg/day |
20 |
mg/kg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
60.0 |
mg/kg |
60 |
mg/kg |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
10.0 |
mg/kg/day |
10 |
mg/kg/day |
intravenous injection, IV |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
10.0 |
mg/kg/day |
10 |
mg/kg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
30.0 |
mg/kg |
30 |
mg/kg |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
20.0 |
mg/kg/day |
20 |
mg/kg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
60.0 |
mg/kg |
60 |
mg/kg |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
10.0 |
mg/kg/day |
10 |
mg/kg/day |
intravenous injection, IV |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
500.0 |
mg/dose |
500 |
mg/dose |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
1500.0 |
mg/dose |
1.5 |
g/dose |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
1000.0 |
mg/day |
1000 |
mg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
2000.0 |
mg/dose |
2 |
g/dose |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for children |
500.0 |
mg/dose |
500 |
mg/dose |
intravenous injection, IV |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
500.0 |
mg/day |
500 |
mg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
1200.0 |
mg/day |
1200 |
mg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
2000.0 |
mg/dose |
2 |
g/dose |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
500.0 |
mg/day |
500 |
mg/day |
intravenous infusion, iv in drop |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
12.0 |
mg/kg/day |
12 |
mg/kg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
30.0 |
mg/kg/dose |
30 |
mg/kg/dose |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
20.0 |
mg/kg/day |
20 |
mg/kg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
1200.0 |
mg/kg |
1200 |
mg/kg |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
60.0 |
mg/kg |
60 |
mg/kg |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
10.0 |
mg/kg/day |
10 |
mg/kg/day |
intravenous injection, IV |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
500.0 |
mg/dose |
500 |
mg/dose |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
1500.0 |
mg/dose |
1.5 |
g/dose |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
1000.0 |
mg/day |
1000 |
mg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
2000.0 |
mg/dose |
2 |
g/dose |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adolescents |
500.0 |
mg/dose |
500 |
mg/dose |
intravenous injection, IV |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adults |
500.0 |
mg/day |
500 |
mg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adults |
1200.0 |
mg/day |
1200 |
mg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adults |
2000.0 |
mg/dose |
2 |
g/dose |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for adults |
500.0 |
mg/day |
500 |
mg/day |
intravenous infusion, iv in drop |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for geriatric |
500.0 |
mg/day |
500 |
mg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for geriatric |
1200.0 |
mg/day |
1200 |
mg/day |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for geriatric |
2.0 |
g/dose |
2 |
g/dose |
PO, oral |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |
| Max dose for geriatric |
500.0 |
mg/day |
500 |
mg/day |
intravenous infusion, iv in drop |
Zithromax 250 mg and 500 mg Tablets and Oral Suspension |
azithromycin |
PDR |