Basic Information
| Drug ID | DDPD00201 |

|
| Drug Name | Caffeine | |
| Molecular Weight | 194.1906 | |
| Molecular Formula | C8H10N4O2 | |
| CAS Number | 58-08-2 | |
| SMILES | CN1C=NC2=C1C(=O)N(C)C(=O)N2C | |
| External Links | ||
| DRUGBANK | DB00201 | |
| T3DB | T3D2712 | |
| PubChem Compound | 2519 | |
| PDR | 3724 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
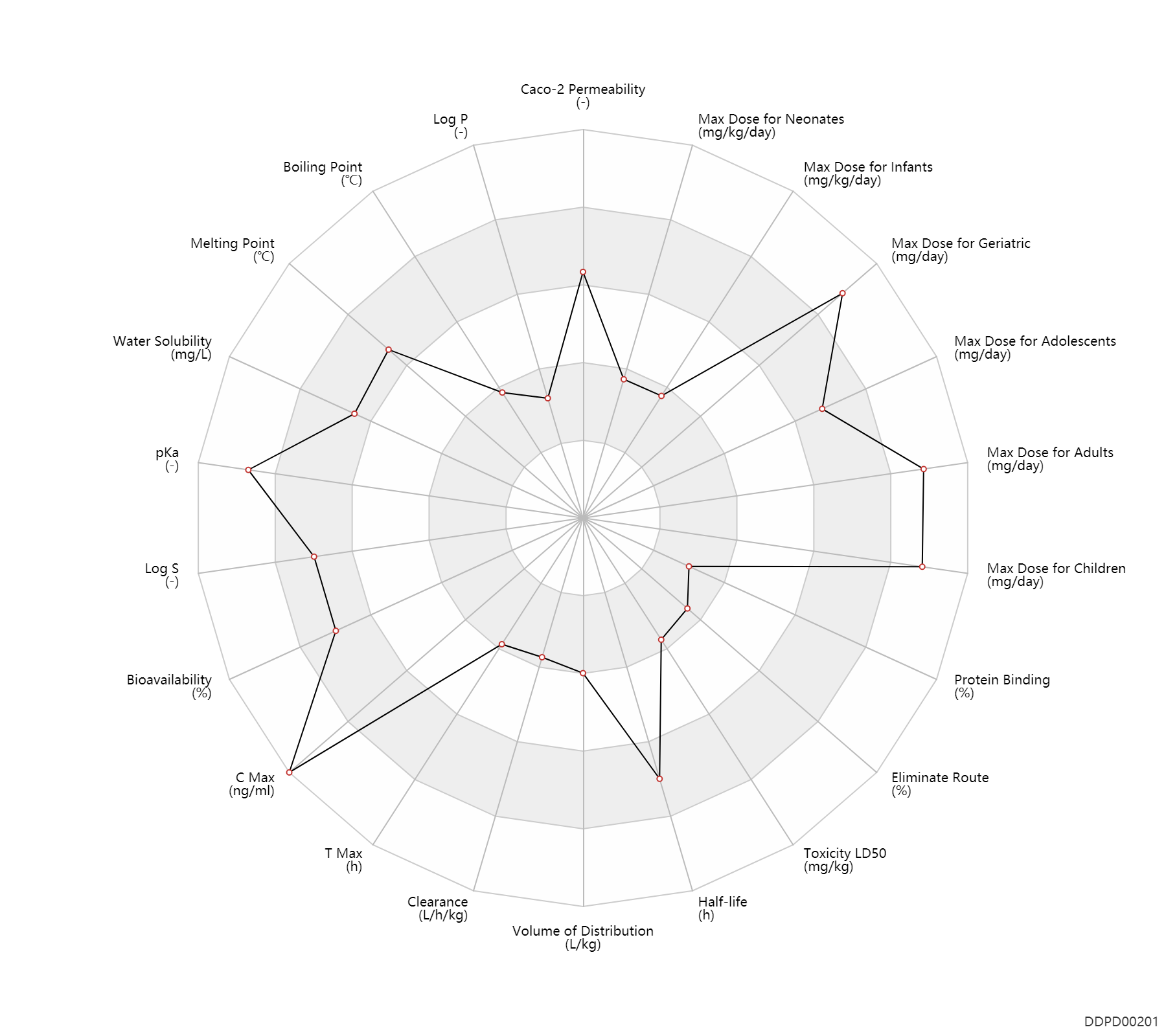
| Caco-2 Permeability | -4.41 | - | -4.41 | - | https://pubs.acs.org/doi/full/10.1021/ci049884m?src=recsys |
| Log P | -0.07 | - | -0.07 | - | http://www.inchem.org/documents/icsc/icsc/eics0405.htm |
| Boiling Point | 178.0 | ℃ | 178 | ℃ | http://www.inchem.org/documents/icsc/icsc/eics0405.htm |
| Melting Point | 236.0 | ℃ | 235-237 | ℃ | https://www.sigmaaldrich.com/catalog/product/sial/41019?lang=en®ion=US |
| Water Solubility | 21700.0 | mg/L | 2.17 | g/100ml | http://www.inchem.org/documents/icsc/icsc/eics0405.htm |
| pKa | 14.0 | - | 14 | - | https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma-Aldrich/Product_Information_Sheet/c0750pis.pdf |
| Log S | -0.97 | - | -0.97 | - | https://pubchem.ncbi.nlm.nih.gov/compound/Caffeine |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 100.0 | % | ~100 | % | PO, oral; adults; | adults ↑ ; | DRUGBANK |
| C Max | 8000.0 | ng/ml | 6-10 | mg/L | PO, oral; | DRUGBANK | |
| T Max | 1.3 | h | 0.5-2 | h | PO, oral; | DRUGBANK | |
| Clearance | 0.0780 | L/h/kg | 1.3 | ml/min/kg | Average clearance; | DRUGBANK | Clearance | 0.0840 | L/h/kg | 1.4 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 0.85 | L/kg | 0.8-0.9 | L/kg | Average volume of distribution; Infants; | DRUGBANK | Volume of Distribution | 0.60 | L/kg | 0.6 | L/kg | Average volume of distribution; adults; | DRUGBANK | Volume of Distribution | 0.63 | L/kg | 0.63 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 5.0 | h | ~5 | h | Children; Adolescents; | DRUGBANK | Half-life | 2.5 | h | ~2.5 | h | Somking; | DRUGBANK | Half-life | 20.0 | h | >20 | h | Preg, pregnant; | DRUGBANK | Half-life | 8.0 | h | ~8 | h | Neonates; | DRUGBANK | Half-life | 100.0 | h | 100 | h | Prem, premature; | hepatopathy,LD ↑ ; | DRUGBANK | Half-life | 4.9 | h | 4.9 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 192.0 | mg/kg | 192.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 127.0 | mg/kg | 127.0 | mg/kg | PO, oral; mouse; | T3DB |
| Toxicity Lethal Dose | 175.0 | mg/kg | ~150-200 | mg/kg | human, homo sapiens; | DRUGBANK | |
| Eliminate Route | 1.3 | % | 0.5-2 | % | Urinary excretion; | DRUGBANK | |
| Protein Binding | 23.0 | % | 10-36 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 3.75 | mg/kg/day | 3.75 | mg/kg/day | PO, oral;intravenous injection, IV; | Caffeine Citrate | caffeine citrate | PDR |
| Max dose for infants | 3.75 | mg/kg/day | 3.75 | mg/kg/day | PO, oral;intravenous injection, IV; | Caffeine Citrate | caffeine citrate | PDR |
| Max dose for children | 1200.0 | mg/day | 1200 | mg/day | PO, oral | Caffeine Citrate | caffeine citrate | PDR |
| Max dose for adolescents | 1200.0 | mg/day | 1200 | mg/day | PO, oral | Caffeine Citrate | caffeine citrate | PDR |
| Max dose for adults | 1200.0 | mg/day | 1200 | mg/day | PO, oral | Caffeine Citrate | caffeine citrate | PDR |
| Max dose for geriatric | 1200.0 | mg/day | 1200 | mg/day | PO, oral | Caffeine Citrate | caffeine citrate | PDR |