Basic Information
| Drug ID | DDPD00191 |

|
| Drug Name | Phentermine | |
| Molecular Weight | 149.2328 | |
| Molecular Formula | C10H15N | |
| CAS Number | 122-09-8 | |
| SMILES | CC(C)(N)CC1=CC=CC=C1 | |
| External Links | ||
| DRUGBANK | DB00191 | |
| T3DB | T3D2709 | |
| PubChem Compound | 4771 | |
| PDR | 23946 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
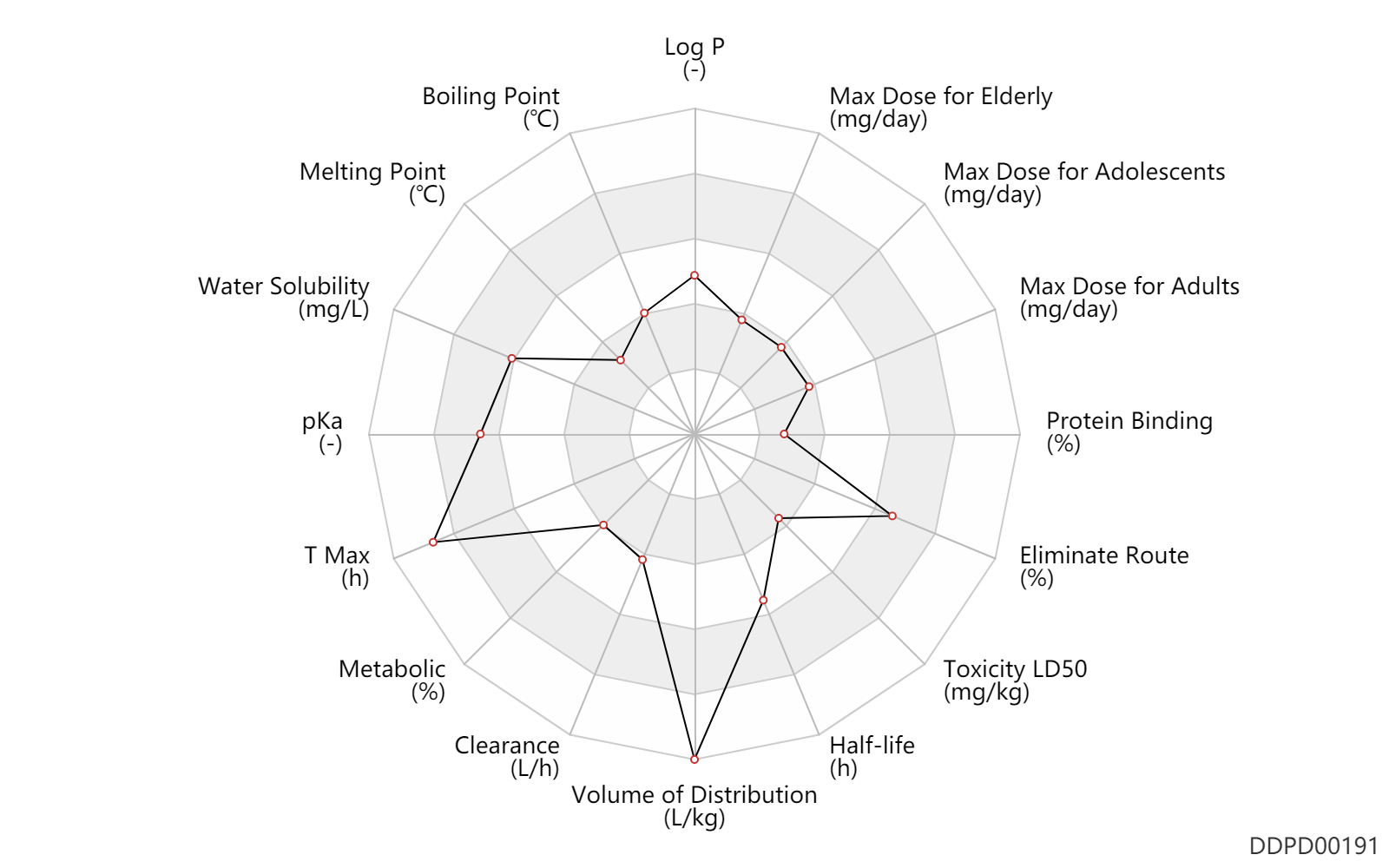
| Log P | 1.9 | - | 1.9 | - | Barceloux D. (2012). Medical Toxicology of Drug Abuse. |
| Boiling Point | 206.0 | ℃ | 206 | ℃ | Barceloux D. (2012). Medical Toxicology of Drug Abuse. |
| Melting Point | 94.5 | ℃ | 205 | ℃ | 'MSDS' |
| Water Solubility | 18600.0 | mg/L | 18.6 | g/L | Gravey W. 2013). Expert Opin Drug Saf. |
| pKa | 9.84 | - | 9.84 | - | Suprenza - package insert. (2014) |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Css | 200.0 | ng/ml | 200.0 | ng/ml | PO, oral; | DRUGBANK |
| T Max | 6.0 | h | 6 | h | PO, oral; | DRUGBANK |
| Metabolic | 6.0 | % | 6.0 | % | DRUGBANK | |
| Clearance | 8.8 | L/h | 8.79 | L/h | PO, oral; | DRUGBANK |
| Volume of Distribution | 59.0 | L/kg | 5.0 | L/kg | DRUGBANK | |
| Half-life | 20.0 | h | ~20 | h | elimination half-life; | DRUGBANK | Half-life | 7.5 | h | 7-8 | h | elimination half-life; Uric acid; | DRUGBANK |
| Toxicity LD50 | 151.0 | mg/kg | 151.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 17.5 | mg/kg | 15-20 | mg/kg | monkey; | T3DB |
| Eliminate Route | 75.0 | % | ~70-80 | % | Urinary excretion; Unchanged drug; | DRUGBANK |
| Protein Binding | 17.5 | % | 17.5 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adolescents | 37.5 | mg/day | 37.5 | mg/day | PO, oral | Lomaira | phentermine hydrochloride | PDR |
| Max dose for adults | 37.5 | mg/day | 37.5 | mg/day | PO, oral | Lomaira | phentermine hydrochloride | PDR |
| Max dose for elderly | 37.5 | mg/day | 37.5 | mg/day | PO, oral | Lomaira | phentermine hydrochloride | PDR |