Basic Information
| Drug ID | DDPD00182 |

|
| Drug Name | Amphetamine | |
| Molecular Weight | 135.2062 | |
| Molecular Formula | C9H13N | |
| CAS Number | 300-62-9 | |
| SMILES | CC(N)CC1=CC=CC=C1 | |
| External Links | ||
| DRUGBANK | DB00182 | |
| T3DB | T3D2706 | |
| PubChem Compound | 3007 | |
| PDR | 3699 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
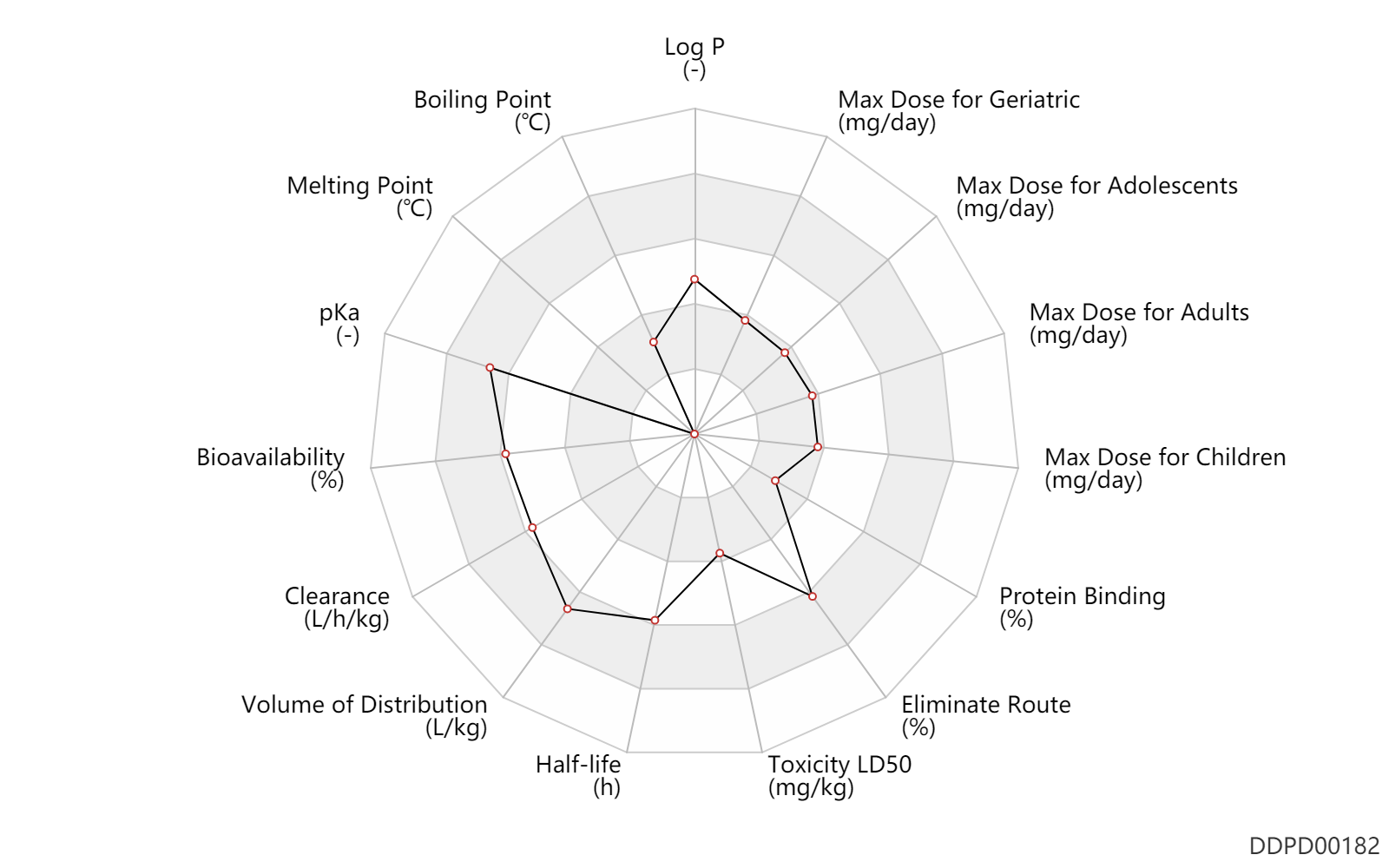
| Log P | 1.76 | - | 1.76 | - | Hooser S., and Khan S. (2018). Elsevier. |
| Boiling Point | 64.7 | ℃ | 64.7 | ℃ | 'MSDS' |
| Melting Point | -98.0 | ℃ | -98 | ℃ | 'MSDS' |
| pKa | 9.9 | - | 9.9 | - | ADDERALL XR (amphetamine) FDA label |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 75.0 | % | >75 | % | PO, oral; intravenous injection, IV; | DRUGBANK | |
| Clearance | 0.70 | L/h/kg | 0.7 | L/h/kg | DRUGBANK | Clearance | 0.40 | L/h/kg | 0.4 | L/h/kg | RD, renal impairment, Renal disease,including uremia ↓ ; | DRUGBANK |
| Volume of Distribution | 4.0 | L/kg | 4.0 | L/kg | DRUGBANK | ||
| Half-life | 10.0 | h | 9-11 | h | Raceme D; | DRUGBANK | Half-life | 12.5 | h | 11-14 | h | Raceme L; | DRUGBANK | Half-life | 7.0 | h | 7 | h | acidity; | DRUGBANK | Half-life | 34.0 | h | 34 | h | alkalinity; | DRUGBANK |
| Toxicity LD50 | 180.0 | mg/kg | 180.0 | mg/kg | subcutaneous injection, SC; Rattus, Rat; | T3DB | |
| Toxicity Lethal Dose | 6.4 | mg/L | 6.4 | mg/L | DRUGBANK | ||
| Eliminate Route | 90.0 | % | ~90 | % | Urinary excretion; PO, oral; | Urine alkaline ↓ ; | DRUGBANK | Eliminate Route | 40.0 | % | ~40 | % | Urinary excretion; Unchanged drug; | Urine alkaline ↓ ; | DRUGBANK |
| Protein Binding | 20.0 | % | 20 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 18.8 | mg/day | 18.8 | mg/day | Tablet,PO,oral | Evekeo | amphetamine sulfate | PDR |
| Max dose for children | 20.0 | mg/day | 20 | mg/day | Liquid | Evekeo | amphetamine sulfate | PDR |
| Max dose for children | 40.0 | mg/day | 40 | mg/day | PO, oral | Evekeo | amphetamine sulfate | PDR |
| Max dose for children | 40.0 | mg/day | 40 | mg/day | PO, oral | Evekeo | amphetamine sulfate | PDR |
| Max dose for adolescents | 12.5 | mg/day | 12.5 | mg/day | Tablet,PO,oral | Evekeo | amphetamine sulfate | PDR |
| Max dose for adolescents | 20.0 | mg/day | 20 | mg/day | Liquid | Evekeo | amphetamine sulfate | PDR |
| Max dose for adolescents | 40.0 | mg/day | 40 | mg/day | PO, oral | Evekeo | amphetamine sulfate | PDR |
| Max dose for adults | 12.5 | mg/day | 12.5 | mg/day | Tablet,PO,oral | Evekeo | amphetamine sulfate | PDR |
| Max dose for adults | 60.0 | mg/day | 60 | mg/day | PO, oral | Evekeo | amphetamine sulfate | PDR |
| Max dose for geriatric | 12.5 | mg/day | 12.5 | mg/day | Tablet,PO,oral | Evekeo | amphetamine sulfate | PDR |
| Max dose for geriatric | 60.0 | mg/day | 60 | mg/day | PO, oral | Evekeo | amphetamine sulfate | PDR |