Basic Information
| Drug ID | DDPD00050 |

|
| Drug Name | Cetrorelix | |
| Molecular Weight | 1431.038 | |
| Molecular Formula | C70H92ClN17O14 | |
| CAS Number | 120287-85-6 | |
| SMILES | CC(C)C[C@H](NC(=O)[C@@H](CCCNC(N)=O)NC(=O)[C@H](CC1=CC=C(O)C=C1)NC(=O)[C@H](CO)NC(=O)[C@@H](CC1=CN=CC=C1)NC(=O)[C@@H](CC1=CC=C(Cl)C=C1)NC(=O)[C@@H](CC1=CC2=CC=CC=C2C=C1)NC(C)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@H](C)C(N)=O | |
| External Links | ||
| DRUGBANK | DB00050 | |
| PubChem Compound | 25074887 | |
| PDR | 1754 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
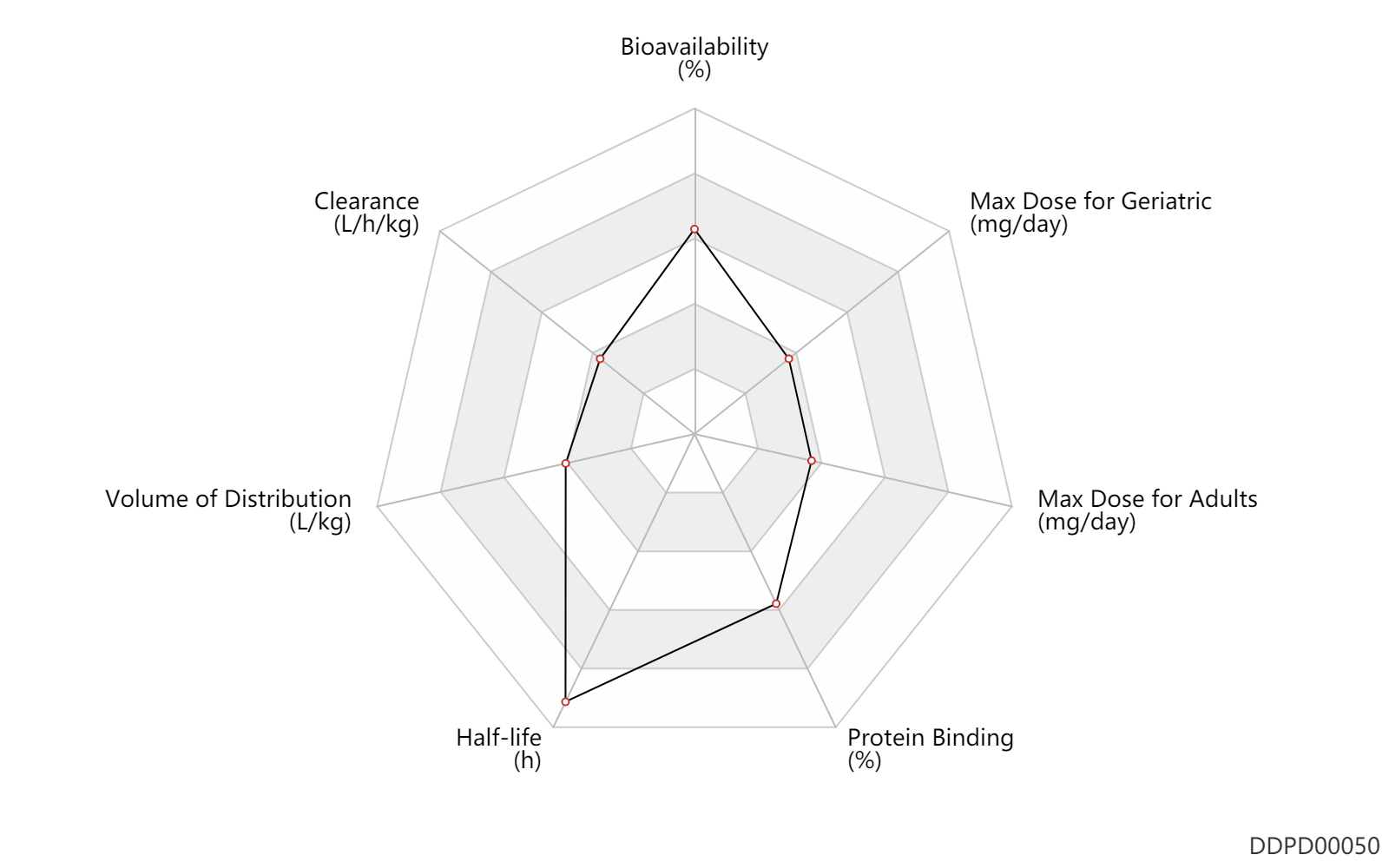
| Bioavailability | 85.0 | % | 85.0 | % | subcutaneous injection, SC; Female, women; | DRUGBANK |
| Clearance | 0.0768 | L/h/kg | 1.28 | ml/min/kg | subcutaneous injection, SC; normal,healthy; Female, women; | DRUGBANK | Clearance | 0.0720 | L/h/kg | 1.2 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 1.2 | L/kg | 1.16 | L/kg | DRUGBANK | Volume of Distribution | 0.39 | L/kg | 0.39 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 62.8 | h | ~62.8 | h | DRUGBANK | Half-life | 12.0 | h | 12 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Protein Binding | 86.0 | % | 86 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 10.0 | mg/day | 10 | mg/day | subcutaneous injection, SC | Cetrotide | cetrorelix acetate | PDR |
| Max dose for geriatric | 10.0 | mg/day | 10 | mg/day | subcutaneous injection, SC | Cetrotide | cetrorelix acetate | PDR |